The daphniphyllum alkaloids: total synthesis of (-)-calyciphylline N
- PMID: 25756504
- PMCID: PMC4394112
- DOI: 10.1021/ja503899t
The daphniphyllum alkaloids: total synthesis of (-)-calyciphylline N
Abstract
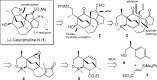
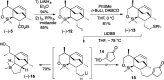
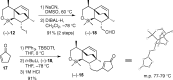
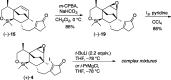
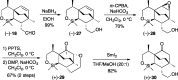
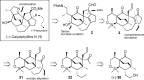
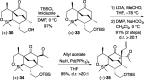
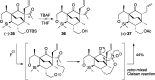
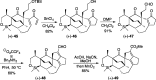
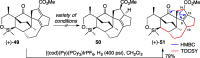
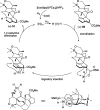
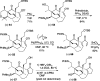
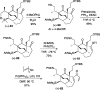
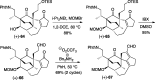
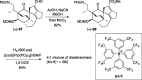
Presented here is a full account on the development of a strategy culminating in the first total synthesis of the architecturally complex daphniphyllum alkaloid, (-)-calyciphylline N. Highlights of the approach include a highly diastereoselective, intramolecular Diels-Alder reaction of a silicon-tethered acrylate; an efficient Stille carbonylation of a sterically encumbered vinyl triflate; a one-pot Nazarov cyclization/proto-desilylation sequence; and the chemoselective hydrogenation of a fully substituted diene ester.
Figures

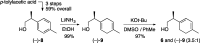










Similar articles
-
Total synthesis of (-)-calyciphylline N.J Am Chem Soc. 2014 Jan 22;136(3):870-3. doi: 10.1021/ja411539w. Epub 2013 Dec 9. J Am Chem Soc. 2014. PMID: 24319987 Free PMC article.
-
Asymmetric synthesis of the tricyclic core of Calyciphylline A-type alkaloids via intramolecular [3 + 2] cycloaddition.Org Lett. 2014 Feb 21;16(4):1076-9. doi: 10.1021/ol403609c. Epub 2014 Feb 7. Org Lett. 2014. PMID: 24506347
-
Calyciphylline B-type Alkaloids: Evolution of a Synthetic Strategy to (-)-Daphlongamine H.J Org Chem. 2019 Nov 1;84(21):14069-14091. doi: 10.1021/acs.joc.9b02223. Epub 2019 Oct 2. J Org Chem. 2019. PMID: 31533427
-
Strategies towards the synthesis of calyciphylline A-type Daphniphyllum alkaloids.Nat Prod Rep. 2014 Apr;31(4):550-62. doi: 10.1039/c3np70115h. Epub 2014 Mar 5. Nat Prod Rep. 2014. PMID: 24595901 Review.
-
Recent Syntheses and Strategies toward Polycyclic Gelsemium Alkaloids.Angew Chem Int Ed Engl. 2019 Jan 14;58(3):681-694. doi: 10.1002/anie.201807509. Epub 2018 Oct 31. Angew Chem Int Ed Engl. 2019. PMID: 30378226 Review.
Cited by
-
The Latest Progress in the Chemistry of Daphniphyllum Alkaloids.Molecules. 2024 Nov 21;29(23):5498. doi: 10.3390/molecules29235498. Molecules. 2024. PMID: 39683658 Free PMC article. Review.
-
Enantioselective Nazarov cyclization of indole enones cooperatively catalyzed by Lewis acids and chiral Brønsted acids.Chem Sci. 2017 Oct 1;8(10):7197-7202. doi: 10.1039/c7sc03183a. Epub 2017 Aug 29. Chem Sci. 2017. PMID: 29081952 Free PMC article.
-
Synthetic studies toward longeracemine: a SmI2-mediated spirocyclization and rearrangement cascade to construct the 2-azabicyclo[2.2.1]heptane framework.Chem Sci. 2020 Jul 30;11(35):9488-9493. doi: 10.1039/d0sc03422c. Chem Sci. 2020. PMID: 34094215 Free PMC article.
-
Tin-Free Access to the ABC Core of the Calyciphylline A Alkaloids and Unexpected Formation of a D-Ring-Contracted Tetracyclic Core.Org Lett. 2018 Apr 20;20(8):2216-2219. doi: 10.1021/acs.orglett.8b00544. Epub 2018 Apr 3. Org Lett. 2018. PMID: 29613805 Free PMC article.
-
Synthesis of Rumphellaone A and Hushinone by a Gold-Catalyzed [2 + 2] Cycloaddition.Org Lett. 2016 Apr 1;18(7):1614-7. doi: 10.1021/acs.orglett.6b00473. Epub 2016 Mar 14. Org Lett. 2016. PMID: 26974011 Free PMC article.
References
-
- Kobayashi J.; Kubota T. Nat. Prod. Rep. 2009, 26, 936. - PubMed
- Kobayashi J.; Morita H. In The Alkaloids; Cordell G. A., Ed.; Academic Press: New York, 2003; Vol. 60, p 165.
- Yamamura S. In The Alkaloids; Brossi A., Ed.; Academic Press: New York, 1986, Vol. 29, p 265.
- Yamamura S.; Hirata Y. In The Alkaloids; Manske R. H. F., Ed.; Academic Press: New York, 1975; Vol. 15, p 41.
-
-
For selected recent examples, see:
- Coldham I.; Burrell A. J. M.; Guerrand H. D. S.; Oram N. Org. Lett. 2011, 13, 1267. - PubMed
- Coldham I.; Watson L.; Adams H.; Martin N. G. J. Org. Chem. 2011, 76, 2360. - PubMed
- Darses B.; Michaelides I. N.; Sladojevich F.; Ward J. W.; Rzepa P. R.; Dixon D. J. Org. Lett. 2012, 14, 1684. - PubMed
- Denmark S. E.; Baiazitov R. Y.; Nguyen S. T. Tetrahedron 2009, 65, 6535. - PMC - PubMed
- Xiong X.; Li Y.; Lu Z.; Wan M.; Deng J.; Wu S.; Shao H.; Li A. Chem. Commun. 2014, 50, 5294. - PubMed
- Yao Y.; Liang G. Org. Lett. 2012, 14, 5499. - PubMed
-
-
- Ruggeri R. B.; Heathcock C. H. Pure Appl. Chem. 1989, 61, 289.
-
- Heathcock C. H.; Hansen M. M.; Ruggeri R. B.; Kath J. C. J. Org. Chem. 1992, 57, 2544.
- Heathcock C. H.; Kath J. C.; Ruggeri R. B. J. Org. Chem. 1995, 60, 1120.
- Ruggeri R. B.; Heathcock C. H. J. Org. Chem. 1990, 55, 3714.
- Stafford J. A.; Heathcock C. H. J. Org. Chem. 1990, 55, 5433.
-
- Weiss M. E.; Carreira E. M. Angew. Chem., Int. Ed. 2011, 50, 11501. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

