Mapping microbial ecosystems and spoilage-gene flow in breweries highlights patterns of contamination and resistance
- PMID: 25756611
- PMCID: PMC4352708
- DOI: 10.7554/eLife.04634
Mapping microbial ecosystems and spoilage-gene flow in breweries highlights patterns of contamination and resistance
Abstract
Distinct microbial ecosystems have evolved to meet the challenges of indoor environments, shaping the microbial communities that interact most with modern human activities. Microbial transmission in food-processing facilities has an enormous impact on the qualities and healthfulness of foods, beneficially or detrimentally interacting with food products. To explore modes of microbial transmission and spoilage-gene frequency in a commercial food-production scenario, we profiled hop-resistance gene frequencies and bacterial and fungal communities in a brewery. We employed a Bayesian approach for predicting routes of contamination, revealing critical control points for microbial management. Physically mapping microbial populations over time illustrates patterns of dispersal and identifies potential contaminant reservoirs within this environment. Habitual exposure to beer is associated with increased abundance of spoilage genes, predicting greater contamination risk. Elucidating the genetic landscapes of indoor environments poses important practical implications for food-production systems and these concepts are translatable to other built environments.
Keywords: beer; built environment; droplet digital PCR; ecology; food fermentation; infectious disease; microbiology; next-generation sequencing; none.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures





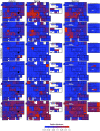

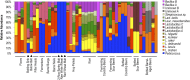

References
-
- Abarenkov K, Henrik Nilsson R, Larsson KH, Alexander IJ, Eberhardt U, Erland S, Høiland K, Kjøller R, Larsson E, Pennanen T, Sen R, Taylor AF, Tedersoo L, Ursing BM, Vrålstad T, Liimatainen K, Peintner U, Kõljalg U. The UNITE database for molecular identification of fungi - recent updates and future perspectives. New Phytologist. 2010;186:281–285. doi: 10.1111/j.1469-8137.2009.03160.x. - DOI - PubMed
-
- Abdo Z, Schüette UM, Bent SJ, Williams CJ, Forney LJ, Joyce P. Statistical methods for characterizing diversity of microbial communities by analysis of terminal restriction fragment length polymorphisms of 16S rRNA genes. Environmental Microbiology. 2006;8:929–938. doi: 10.1111/j.1462-2920.2005.00959.x. - DOI - PubMed
-
- Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecology. 2001;26:32–46.
-
- Back W. Beer spoilage bacteria. Taxonomy of beer spoilage bacteria. Gram positive species. Monatsschr Brauwiss. 1981;34:267–276.

