Herpes simplex type 2 virus deleted in glycoprotein D protects against vaginal, skin and neural disease
- PMID: 25756612
- PMCID: PMC4352706
- DOI: 10.7554/eLife.06054
Herpes simplex type 2 virus deleted in glycoprotein D protects against vaginal, skin and neural disease
Abstract
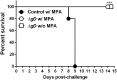
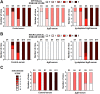
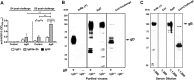
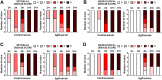
Subunit vaccines comprised of glycoprotein D (gD-2) failed to prevent HSV-2 highlighting need for novel strategies. To test the hypothesis that deletion of gD-2 unmasks protective antigens, we evaluated the efficacy and safety of an HSV-2 virus deleted in gD-2 and complemented allowing a single round of replication on cells expressing HSV-1 gD (ΔgD(-/+gD-1)). Subcutaneous immunization of C57BL/6 or BALB/c mice with ΔgD(-/+gD1) provided 100% protection against lethal intravaginal or skin challenges and prevented latency. ΔgD(-/+gD1) elicited no disease in SCID mice, whereas 1000-fold lower doses of wild-type virus were lethal. HSV-specific antibodies were detected in serum (titer 1:800,000) following immunization and in vaginal washes after intravaginal challenge. The antibodies elicited cell-mediated cytotoxicity, but little neutralizing activity. Passive transfer of immune serum completely protected wild-type, but not Fcγ-receptor or neonatal Fc-receptor knock-out mice. These studies demonstrate that non-neutralizing Fc-mediated humoral responses confer protection and support advancement of this attenuated vaccine.
Keywords: HSV-2; antibody; immunity; infectious disease; microbiology; mouse; vaccine; virology.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures








References
-
- Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, Gorfinkel I, Morrow RL, Ewell MG, Stokes-Riner A, Dubin G, Heineman TC, Schulte JM, Deal CD, Herpevac Trial for Women Efficacy results of a trial of a herpes simplex vaccine. The New England Journal of Medicine. 2012;366:34–43. doi: 10.1093/infdis/jit651. - DOI - PMC - PubMed
-
- Bernstein DI, Aoki FY, Tyring SK, Stanberry LR, St-Pierre C, Shafran SD, Leroux-Roels G, Van Herck K, Bollaerts A, Dubin G, GlaxoSmithKline Herpes Vaccine Study Group Safety and immunogenicity of glycoprotein D-adjuvant genital herpes vaccine. Clinical Infectious Diseases. 2005;40:1271–1281. doi: 10.1086/429240. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

