Bidentate, monoanionic auxiliary-directed functionalization of carbon-hydrogen bonds
- PMID: 25756616
- PMCID: PMC4406856
- DOI: 10.1021/ar5004626
Bidentate, monoanionic auxiliary-directed functionalization of carbon-hydrogen bonds
Abstract
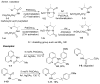
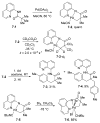
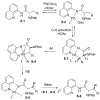
In recent years, carbon-hydrogen bond functionalization has evolved from an organometallic curiosity to a tool used in mainstream applications in the synthesis of complex natural products and drugs. The use of C-H bonds as a transformable functional group is advantageous because these bonds are the most abundant functionality in organic molecules. One-step conversion of these bonds to the desired functionality shortens synthetic pathways, saving reagents, solvents, and labor. Less chemical waste is generated as well, showing that this chemistry is environmentally beneficial. This Account describes the development and use of bidentate, monoanionic auxiliaries for transition-metal-catalyzed C-H bond functionalization reactions. The chemistry was initially developed to overcome the limitations with palladium-catalyzed C-H bond functionalization assisted by monodentate directing groups. By the use of electron-rich bidentate directing groups, functionalization of unactivated sp(3) C-H bonds under palladium catalysis has been developed. Furthermore, a number of abundant base-metal complexes catalyze functionalization of sp(2) C-H bonds. At this point, aminoquinoline, picolinic acid, and related compounds are among the most used and versatile directing moieties in C-H bond functionalization chemistry. These groups facilitate catalytic functionalization of sp(2) and sp(3) C-H bonds by iron, cobalt, nickel, copper, ruthenium, rhodium, and palladium complexes. Exceptionally general reactivity is observed, enabling, among other transformations, direct arylation, alkylation, fluorination, sulfenylation, amination, etherification, carbonylation, and alkenylation of carbon-hydrogen bonds. The versatility of these auxilaries can be attributed to the following factors. First, they are capable of stabilizing high oxidation states of transition metals, thereby facilitating the C-H bond functionalization step. Second, the directing groups can be removed, enabling their use in synthesis and functionalization of natural products and medicinally relevant substances. While the development of these directing groups presents a significant advance, several limitations of this methodology are apparent. The use of expensive second-row transition metal catalysts is still required for efficient sp(3) C-H bond functionalization. Furthermore, the need to install and subsequently remove the relatively expensive directing group is a disadvantage.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
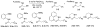
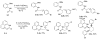



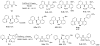
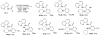

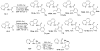


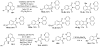
References
-
- Engle KM, Mei TS, Wang X, Yu JQ. Bystanding F+ Oxidants Enable Selective Reductive Elimination from High-Valent Metal Centers in Catalysis. Angew Chem, Int Ed. 2011;50:1478–1491. - PMC - PubMed
- Lyons TW, Sanford MS. Palladium-Catalyzed Ligand-Directed C–H Functionalization Reactions. Chem Rev. 2010;110:1147–1169. - PMC - PubMed
- Gutekunst WR, Baran PS. C–H Functionalization Logic in Total Synthesis. Chem Soc Rev. 2011;40:1976–1991. - PubMed
- Ackermann L. Carboxylate-Assisted Transition-Metal-Catalyzed C–H Bond Functionalizations: Mechanism and Scope. Chem Rev. 2011;111:1315–1345. - PubMed
- Baudoin O. Transition Metal-Catalyzed Arylation of Unactivated C(sp3)–H Bonds. Chem Soc Rev. 2011;40:4902–4911. - PubMed
- Ge K, Yoshikai N. Low-Valent Cobalt Catalysis: New Opportunities for C–H Functionalization. Acc Chem Res. 2014;47:1208–1219. - PubMed
- Rouquet G, Chatani N. Catalytic Functionalization of C(sp2)–H and C(sp3)–H Bonds by Using Bidentate Directing Groups. Angew Chem, Int Ed. 2013;52:11726–11743. - PubMed
- Colby DA, Bergman RG, Ellman JA. Rhodium-Catalyzed C–C Bond Formation via Heteroatom-Directed C–H Bond Activation. Chem Rev. 2010;110:624–655. - PMC - PubMed
- Zhang F, Spring DR. Arene C–H Functionalisation Using a Removable or Traceless Directing Group Strategy. Chem Soc Rev. 2014;43:6906–6919. - PubMed
-
- Arndtsen BA, Bergman RG, Mobley TA, Peterson TH. Selective Intermolecular Carbon–Hydrogen Bond Activation by Synthetic Metal Complexes in Homogeneous Solution. Acc Chem Res. 1995;28:154–162.
-
- Vogel A., Jr . Kunst-und Gewerbeblatt des Polytechnischen Vereins für das Konigreich Bayern. 1858. Chemisch-technische Mittheilungen; pp. 23–33.
- Quet M. Note sur un phénomène de polarité dans la décomposition des gaz par l’étincelle électrique. C R Hebd Seances Acad Sci. 1858;46:903–905.
- Glaser C. Beiträge zur Kenntniss des Acetenylbenzolsenylbenzols. Ber Dtsch Chem Ges. 1869;2:422–424.
-
- Volhard J. Ueber Verbindungen des Thiophens, seiner Homologen und einiger Ketone mit Quecksilberchlorid. Liebigs Ann Chem. 1892;267:172–185.
-
- Kharasch MS, Isbell HS. The Chemistry of Organic Gold Compounds. III. Direct Introduction of Gold into the Aromatic Nucleus. J Am Chem Soc. 1931;53:3053–3059.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

