LC-ESI-MS/MS analysis and pharmacokinetics of GP205, an innovative potent macrocyclic inhibitor of hepatitis C virus NS3/4A protease in rats
- PMID: 25756650
- PMCID: PMC6272426
- DOI: 10.3390/molecules20034319
LC-ESI-MS/MS analysis and pharmacokinetics of GP205, an innovative potent macrocyclic inhibitor of hepatitis C virus NS3/4A protease in rats
Abstract
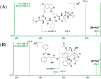
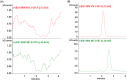
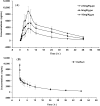
A high-throughput, sensitive and specific LC-ESI-MS/MS method was established for the quantitative determination of GP205, a potent inhibitor of hepatitis C virus NS3/4A protease, in rat. The analyte was isolated from 25 μL plasma sample by 96-well LLE. Good linearity was achieved within the concentration range of 2-5000 ng/mL (r2 > 0.996). The intra- and inter-day precision was less than 10%. The accuracy ranged from 0.8% to 5.5% for GP205 in quality control samples at three levels. GP205 was stable during the analysis and the storage period. The method was successfully applied to pharmacokinetic studies of GP205 in Sprague-Dawley rats. The pharmacokinetic profiles of GP205 at three dose levels with oral administration and one dose level with intravenous administration were successfully studied for the first time in SD rats, respectively. After single oral administration of GP205 at the doses of 2.5, 5, 10 mg/kg, respectively, Cmax and AUC0-τ were proportional to the doses given. The absolute bioavailability was estimated as 34% based on the AUCs of oral administration at the dose of 5 mg/kg and intravenous administration at the dose of 1 mg/kg. The data presented in this study provides useful information for further study for GP205.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hepatitis C—Global prevalence (update) Wkly. Epidemiol. Rec. 1999;74:425–427. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

