Positive pressure therapy for Ménière's disease or syndrome
- PMID: 25756795
- PMCID: PMC11026870
- DOI: 10.1002/14651858.CD008419.pub2
Positive pressure therapy for Ménière's disease or syndrome
Abstract
Background: Ménière's disease is an incapacitating disease in which recurrent attacks of vertigo are accompanied by hearing loss, tinnitus and/or aural fullness, all of which are discontinuous and variable in intensity. A number of different therapies have been identified for patients with this disease, ranging from dietary measures (e.g. a low-salt diet) and medication (e.g. betahistine (Serc®), diuretics) to extensive surgery (e.g. endolymphatic sac surgery). The Meniett® low-pressure pulse generator (Medtronic ENT, 1999) is a device that is designed to generate a computer-controlled sequence of low-pressure (micro-pressure) pulses, which are thought to be transmitted to the vestibular system of the inner ear. The pressure pulse passes via a tympanostomy tube (grommet) to the middle ear, and hence to the inner ear via the round and/or oval window. The hypothesis is that these low-pressure pulses reduce endolymphatic hydrops.
Objectives: To assess the effects of positive pressure therapy (e.g. the Meniett device) on the symptoms of Ménière's disease or syndrome.
Search methods: We searched the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL); PubMed; EMBASE; CINAHL; Web of Science; Cambridge Scientific Abstracts; ICTRP and additional sources for published and unpublished trials. The date of the search was 6 June 2014.
Selection criteria: Randomised controlled trials (RCTs) comparing positive pressure therapy (using the Meniett or a similar device) with placebo in patients with Ménière's disease. The primary outcome was control of vertigo; secondary outcomes were loss or gain of hearing, severity of tinnitus, perception of aural fullness, functional level, complications or adverse effects, and sick days.
Data collection and analysis: Two authors independently selected studies, assessed risk of bias and extracted data. We contacted authors for additional data. Where possible, we pooled study results using a fixed-effect, mean difference (MD) meta-analysis and tested for statistical heterogeneity using both the Chi² test and I² statistic. This was only possible for the secondary outcomes loss or gain of hearing and sick days. We presented results using forest plots with 95% confidence intervals (Cl).
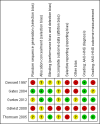
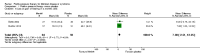
Main results: We included five randomised clinical trials with 265 participants. All trials were prospective, double-blind, placebo-controlled randomised controlled trials on the effects of positive pressure therapy on vertigo complaints in Ménière's disease. Overall, the risk of bias varied: three out of five studies were at low risk, one was at unclear risk and one was at high risk of bias. Control of vertigo For the primary outcome, control of vertigo, it was not possible to pool data due to heterogeneity in the measurement of the outcome measures. In most studies, no significant difference was found between the positive pressure therapy group and the placebo group in vertigo scores or vertigo days. Only one study, at low risk of bias, showed a significant difference in one measure of vertigo control in favour of positive pressure therapy. In this study, the mean visual analogue scale (VAS) score for vertigo after eight weeks of treatment was 25.5 in the positive pressure therapy group and 46.6 in the placebo group (mean difference (MD) -21.10, 95% CI -35.47 to -6.73; scale not stated - presumed to be 0 to 100). Secondary outcomes For the secondary outcomes, we carried out two pooled analyses. We found statistically significant results for loss or gain of hearing . Hearing was 7.38 decibels better in the placebo group compared to the positive pressure therapy group (MD) (95% CI 2.51 to 12.25; two studies, 123 participants). The severity of tinnitus and perception of aural fullness were either not measured or inadequate data were provided in the included studies. For the secondary outcome functional level , it was not possible to perform a pooled analysis. One included study showed less functional impairment in the positive pressure group than the placebo group (AAO-HNS criteria, one- to six-point scale: MD -1.10, 95% CI -1.81 to -0.39, 40 participants); another study did not show any significant results. In addition to the predefined secondary outcome measures, we included sick days as an additional outcome measure, as two studies used this outcome measure and it is a complementary measurement of impairment due to Ménière's disease. We did not find a statistically significant difference in sick days. No complications or adverse effects were noted by any study.
Authors' conclusions: There is no evidence, from five included studies, to show that positive pressure therapy is effective for the symptoms of Ménière's disease. There is some moderate quality evidence, from two studies, that hearing levels are worse in patients who use this therapy. The positive pressure therapy device itself is minimally invasive. However, in order to use it, a tympanostomy tube (grommet) needs to be inserted, with the associated risks. These include the risks of anaesthesia, the general risks of any surgery and the specific risks of otorrhoea and tympanosclerosis associated with the insertion of a tympanostomy tube. Notwithstanding these comments, no complications or adverse effects were noted in any of the included studies.
Conflict of interest statement
Sanne van Sonsbeek: none known. Bas Pullens: none known Peter Paul van Benthem: none known.
Figures
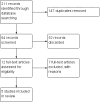









Update of
References
References to studies included in this review
Densert 1997 {published data only}
-
- Densert B, Densert O, Arlinger S, Sass K, Odkvist L. Immediate effects of middle ear pressure changes on the electrocochleographic recordings in patients with Ménière's disease: a clinical placebo‐controlled study. American Journal of Otology 1997;18:726‐33. - PubMed
Gates 2004 {published data only}
-
- Gates GA, Green JD Jr, Tucci DL, Telian SA. The effects of transtympanic micropressure treatment in people with unilateral Ménière's disease. Archives of Otolaryngology ‐‐ Head and Neck Surgery 2004;130(6):718‐25. [PUBMED: 15210552] - PubMed
Gurkov 2012 {published data only}
-
- Gurkov R, Filipe Mingas LB, Rader T, Louza J, Olzowy B, Krause E. Effects of transtympanic low‐pressure therapy in patients with unilateral Ménière's disease unresponsive to betahistine: a randomised, placebo‐controlled, double‐blind, clinical trial. Journal of Laryngology & Otology 2012;126:356‐62. - PubMed
Odkvist 2000 {published data only}
-
- Odkvist LM, Arlinger S, Billermark E, Densert B, Lindholm S, Wallqvist J. Effects of middle ear pressure changes on clinical symptoms in patients with Ménière's Disease ‐ a clinical multicentre placebo‐controlled study. Acta Oto‐Laryngologica. Supplement 2000;543:99‐101. - PubMed
Thomsen 2005 {published data only}
-
- Thomsen J, Sass K, Odkvist L, Arlinger S. Local overpressure treatment reduces vestibular symptoms in patients with Ménière's disease: a clinical, randomised, multicenter, double‐blind, placebo‐controlled study. Otology & Neurotology 2005;26(1):68‐73. [PUBMED: 15699722] - PubMed
References to studies excluded from this review
Barbara 2001 {published data only}
-
- Barbara M, Consagra C, Monini S, Nostro G, Harguindey A, Vestri A, et al. Local pressure protocol, including Meniett, in the treatment of Meniere's disease: short‐term results during the active stage. Acta Oto‐Laryngologica 2001;121:939‐44. - PubMed
Buchanan 2010 {published data only}
-
- Buchanan M, Rai A, Prinsley P. Initial UK experience of patient satisfaction with the Meniett device for Meniere's disease treatment. Journal of Laryngology and Otology 2010;124:1067‐72. - PubMed
Densert 2001 {published data only}
-
- Densert B, Sass K. Control of symptoms in patients with Ménière's disease using middle ear pressure applications: two year follow‐up. Acta Oto‐Laryngologica 2001;121:616‐21. - PubMed
Franz 2005 {published data only}
-
- Franz B. P‐100 in the treatment of Meniere's disease: a clinical study. International Tinnitus Journal 2005;11:146‐9. - PubMed
Shojaku 2011 {published data only}
-
- Shojaku H, Watanabe Y, Mineta H, Aoki M, Tsubota M, Watanabe K, et al. Long‐term effects of the Meniett device in Japanese patients with Meniere's disease and delayed endolymphatic hydrops reported by the Middle Ear Pressure Treatment Research Group of Japan. Acta Oto‐Laryngologica 2011;131:277‐83. - PubMed
Stokroos 2006 {published data only}
-
- Stokroos R, Klein Olvink M, Hendrice N, Kingma H. Functionality outcome of treatment of Meniere's disease with the Meniett pressure generator. Acta Oto‐Laryngologica 2006;126:254‐8. - PubMed
Watanabe 2011 {published data only}
-
- Watanabe Y, Shojaku H, Junicho M, Asai M, Fujisaka M, Takakura H, et al. Intermittent pressure therapy of intractable Meniere's disease and delayed endolymphatic hydrops using the transtympanic membrane massage device: a preliminary report. Acta Oto‐Laryngologica 2011;131:1178‐86. - PubMed
Additional references
AAO‐HNS 1995
-
- Committee on Hearing and Equilibrium. Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Menière's disease. American Academy of Otolaryngology ‐ Head and Neck Foundation, Inc. Otolaryngology ‐ Head and Neck Surgery 1995;113(3):181‐5. - PubMed
Ahsan 2013
-
- Ahsan SF, Standring RT. Meta‐analysis of Meniett therapy for Meniere's disease. Otolaryngology ‐ Head and Neck Surgery 2013;149(2 Suppl):P105.
Alford 1972
-
- Alford BR. Menière's disease: criteria for diagnosis and evaluation of therapy for reporting. Report of subcommittee on equilibrium and its measurement. Transactions of the American Academy of Ophthalmology and Otolaryngology 1972;76:1462‐4. - PubMed
Balkany 1980
-
- Balkany TJ, Sires B, Arenberg IK. Bilateral aspects of Meniere's disease: an underestimated clinical entity. Otolaryngologic Clinics of North America 1980;13(4):603‐9. - PubMed
Burgess 2006
Coelho 2008
-
- Coelho DH, Lalwani AK. Medical management of Meniere's disease. Laryngoscope 2008;118(6):1099‐108. - PubMed
Densert 1982
-
- Densert B, Densert O. Overpressure in treatment of Meniere's disease. Laryngoscope 1982;92(11):1285‐92. [PUBMED: 6983015] - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. - PubMed
Durland 2005
-
- Durland WF Jr, Pyle GM, Connor NP. Endolymphatic sac decompression as a treatment for Meniere's disease. Laryngoscope 2005;115(8):1454‐7. - PubMed
Egami 1978
-
- Egami T, Sando I, Black FO. Hypoplasia of the vestibular aqueduct and endolymphatic sac in endolymphatic hydrops. Otolaryngology 1978;86(2):327‐39. [PUBMED: 113739] - PubMed
Feijen 2000
-
- Feijen RA, Segenhout JM, Wit HP, Albers FW. Monitoring inner ear pressure changes in normal guinea pigs induced by the Meniett20. Acta Oto‐Laryngologica 2000;120(7):804‐9. - PubMed
Feijen 2002
-
- Feijen RA, Segenhout JM, Albers FW, Wit HP. Change of guinea pig inner ear pressure by square wave middle ear cavity pressure variation. Acta Oto‐Laryngologica 2002;122(2):138‐45. [PUBMED: 11936904] - PubMed
Gates 2006
-
- Gates GA, Verrall A, Green JD, Tucci DL, Telian SA. Meniett clinical trial: long‐term follow up. Archives of Otolaryngology ‐‐ Head and Neck Surgery 2006;132:1311‐6. - PubMed
Ghossaini 2006
-
- Ghossaini SN, Wazen JJ. An update on the surgical treatment of Meniere's diseases. Journal of the American Academy of Audiology 2006;17(1):38‐44. - PubMed
Gibson 1991
-
- Gibson WPR. The 10‐point score for the clinical diagnosis of Ménière's disease. Proceedings of the Third International Symposium and Workshops on the Surgery of the Inner Ear. Amsterdam: Kugler Publications, 1991:109.
Hallpike 1938
Handbook 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ingelstedt 1976
-
- Ingelstedt S, Ivarsson A, Tjernstrom O. Immediate relief of symptoms during acute attacks of Meniere's disease, using a pressure chamber. Acta Oto‐Laryngologica 1976;82(5‐6):368‐78. - PubMed
James 2001
Kimura 1967
-
- Kimura RS. Experimental blockage of the endolymphatic duct and sac and its effect on the inner ear of the guinea pig. A study on endolymphatic hydrops. Annals of Otology, Rhinology, and Laryngology 1967;76(3):664‐87. - PubMed
Kitahara 1991
-
- Kitahara M. Bilateral aspects of Meniere's disease. Meniere's disease with bilateral fluctuant hearing loss. Acta Oto‐Laryngologica. Supplement 1991;485:74‐7. - PubMed
Kotimaki 1999
-
- Kotimaki J, Sorri M, Aantaa E, Nuutinen J. Prevalence of Meniere disease in Finland. Laryngoscope 1999;109(5):748‐53. - PubMed
Mantel 1959
-
- Mantel N, Haensxel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute 1959;22:719‐48. - PubMed
Medtronic ENT
-
- Treatment of Meniere's disease with the Meniett low‐pressure pulse generator. An independent clinical studies review for Medtronic ENT. http://www.medtronic.com/wcm/groups/mdtcom_sg/@mdt/@ent/documents/docume... 2012.
Meniere 1861
-
- Ménière P. Memoire sur les lesions de l'oreille interne donnant lieu à des symptômes de congestion cerebrale apoplectiforme. Gazette Medicale de Paris 1861;16:597‐601.
Monsell 1995
-
- Monsell EM. New and revised reporting guidelines from the Committee on Hearing and Equilibrium. American Academy of Otolaryngology ‐ Head and Neck Surgery Foundation, Inc. Otolaryngology ‐ Head and Neck Surgery 1995;113(3):176‐8. - PubMed
Morrison 1995
-
- Morrison AW. Anticipation in Meniere's disease. Journal of Laryngology and Otology 1995;109(6):499‐502. - PubMed
Nakae 1984
-
- Nakae K, Komatuzaki K. Epidemiological study of Meniere's disease. Pract Otol (Kyoto) 1984;69:1783‐8.
NHS 2012
-
- Meniett low‐pressure pulse generator (Medtronic ENT) for treatment of Meniere's disease. Healthcare Technology Brief Publication. Lansdale: HAYES, Inc 2012.
Odkvist 2001
-
- Odkvist L. Pressure treatment versus gentamicin for Meniere's disease. Acta Oto‐Laryngologica 2001;121(2):266‐8. - PubMed
Pearson 1985
-
- Pearson BW, Brackmann DE. Committee on Hearing and Equilibrium guidelines for reporting treatment results in Meniere's disease. Otolaryngology ‐ Head and Neck Surgery 1985;93(5):579‐81. - PubMed
Portmann 1980
-
- Portmann G. The old and new in Meniere's disease ‐ over 60 years in retrospect and a look to the future. Otolaryngologic Clinics of North America 1980;13(4):567‐75. - PubMed
Pullens 2013
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sajjadi 2008
-
- Sajjadi H, Paparella MM. Meniere's disease. Lancet 2008;372(9636):406‐14. - PubMed
Sakikawa 1997
-
- Sakikawa Y, Kimura RS. Middle ear overpressure treatment of endolymphatic hydrops in guinea pigs. ORL; Journal for Oto‐rhino‐laryngology and its Related Specialties 1997;59(2):84‐90. - PubMed
Silverstein 1989
-
- Silverstein H, Smouha E, Jones R. Natural history vs. surgery for Meniere's disease. Otolaryngology ‐ Head and Neck Surgery 1989;100(1):6‐16. - PubMed
Stahle 1978
-
- Stahle J, Stahle C, Arenberg IK. Incidence of Meniere's disease. Archives of Otolaryngology 1978;104(2):99‐102. - PubMed
Tjernstrom 1977
-
- Tjernstrom O. Effects of middle ear pressure on the inner ear. Acta Oto‐Laryngologica 1977;83(1‐2):11‐5. - PubMed
Vrabec 2003
-
- Vrabec JT. Herpes simplex virus and Meniere's disease. Laryngoscope 2003;113(9):1431‐8. - PubMed
Yamakawa 1938
-
- Yamakawa K. Uber die pathologische Veranderung bei einem Menière‐Kranken. Proceedings of the 42nd Annual Meeting of the the Oto‐Rhino‐Laryngological Society of Japan 1938;44:2310‐2.
Yamamoto 1992
-
- Yamamoto E, Mizukami C, Ohmura M. Investigation of the external aperture of the vestibular aqueduct in Meniere's disease by three‐dimensional image analysis. Acta Oto‐Laryngologica 1992;112(1):31‐5. [PUBMED: 1575034] - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

