Melanoma Cell Galectin-1 Ligands Functionally Correlate with Malignant Potential
- PMID: 25756799
- PMCID: PMC4466041
- DOI: 10.1038/jid.2015.95
Melanoma Cell Galectin-1 Ligands Functionally Correlate with Malignant Potential
Abstract
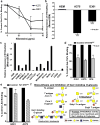
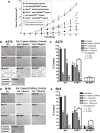
Galectin-1 (Gal-1)-binding to Gal-1 ligands on immune and endothelial cells can influence melanoma development through dampening antitumor immune responses and promoting angiogenesis. However, whether Gal-1 ligands are functionally expressed on melanoma cells to help control intrinsic malignant features remains poorly understood. Here, we analyzed expression, identity, and function of Gal-1 ligands in melanoma progression. Immunofluorescent analysis of benign and malignant human melanocytic neoplasms revealed that Gal-1 ligands were abundant in severely dysplastic nevi, as well as in primary and metastatic melanomas. Biochemical assessments indicated that melanoma cell adhesion molecule (MCAM) was a major Gal-1 ligand on melanoma cells that was largely dependent on its N-glycans. Other melanoma cell Gal-1 ligand activity conferred by O-glycans was negatively regulated by α2,6 sialyltransferase ST6GalNAc2. In Gal-1-deficient mice, MCAM-silenced (MCAM(KD)) or ST6GalNAc2-overexpressing (ST6(O/E)) melanoma cells exhibited slower growth rates, underscoring a key role for melanoma cell Gal-1 ligands and host Gal-1 in melanoma growth. Further analysis of MCAM(KD) or ST6(O/E) melanoma cells in cell migration assays indicated that Gal-1 ligand-dependent melanoma cell migration was severely inhibited. These findings provide a refined perspective on Gal-1/melanoma cell Gal-1 ligand interactions as contributors to melanoma malignancy.
Conflict of interest statement
While all other authors declare no conflicts of interest, Dr. Abrar A. Qureshi consults for Abbvie, Inc., Amgen, Inc., Novartis Corp, the Centers for Disease Control and Prevention, Janssen, Inc. and Merck & Co., Inc., and serves as an investigator for Amgen, Inc.
Figures




References
-
- Andre S, Kojima S, Yamazaki N, et al. Galectins-1 and -3 and their ligands in tumor biology. Non-uniform properties in cell-surface presentation and modulation of adhesion to matrix glycoproteins for various tumor cell lines, in biodistribution of free and liposome-bound galectins and in their expression by breast and colorectal carcinomas with/without metastatic propensity. Journal of cancer research and clinical oncology. 1999;125:461–74. - PubMed
-
- Baum LG, Seilhamer JJ, Pang M, et al. Synthesis of an endogeneous lectin, galectin-1, by human endothelial cells is up-regulated by endothelial cell activation. Glycoconjugate journal. 1995;12:63–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

