Exposure to Leishmania braziliensis triggers neutrophil activation and apoptosis
- PMID: 25756874
- PMCID: PMC4354905
- DOI: 10.1371/journal.pntd.0003601
Exposure to Leishmania braziliensis triggers neutrophil activation and apoptosis
Abstract
Background: Neutrophils are the first line of defense against invading pathogens and are rapidly recruited to the sites of Leishmania inoculation. During Leishmania braziliensis infection, depletion of inflammatory cells significantly increases the parasite load whereas co-inoculation of neutrophils plus L. braziliensis had an opposite effect. Moreover, the co-culture of infected macrophages and neutrophils also induced parasite killing leading us to ask how neutrophils alone respond to an L. braziliensis exposure. Herein we focused on understanding the interaction between neutrophils and L. braziliensis, exploring cell activation and apoptotic fate.
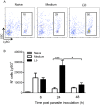
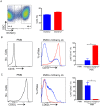
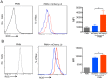
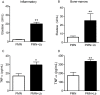
Methods and findings: Inoculation of serum-opsonized L. braziliensis promastigotes in mice induced neutrophil accumulation in vivo, peaking at 24 h. In vitro, exposure of thyoglycollate-elicited inflammatory or bone marrow neutrophils to L. braziliensis modulated the expression of surface molecules such as CD18 and CD62L, and induced the oxidative burst. Using mCherry-expressing L. braziliensis, we determined that such effects were mainly observed in infected and not in bystander cells. Neutrophil activation following contact with L. braziliensis was also confirmed by the release of TNF-α and neutrophil elastase. Lastly, neutrophils infected with L. braziliensis but not with L. major displayed markers of early apoptosis.
Conclusions: We show that L. braziliensis induces neutrophil recruitment in vivo and that neutrophils exposed to the parasite in vitro respond through activation and release of inflammatory mediators. This outcome may impact on parasite elimination, particularly at the early stages of infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Nathan C (2006) Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol 6: 173–182. - PubMed
-
- Babior BM (1999) NADPH oxidase: an update. Blood 93: 1464–1476. - PubMed
-
- Borregaard N, Sorensen OE, Theilgaard-Monch K (2007) Neutrophil granules: a library of innate immunity proteins. Trends Immunol 28: 340–345. - PubMed
-
- Savill J, Haslett C (1995) Granulocyte clearance by apoptosis in the resolution of inflammation. Semin Cell Biol 6: 385–393. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

