Clinical setting influences off-label and unlicensed prescribing in a paediatric teaching hospital
- PMID: 25756896
- PMCID: PMC4355417
- DOI: 10.1371/journal.pone.0120630
Clinical setting influences off-label and unlicensed prescribing in a paediatric teaching hospital
Abstract
Purpose: To estimate the prevalence of off-label and unlicensed prescribing during 2008 at a major paediatric teaching hospital in Western Australia.
Methods: A 12-month retrospective study was conducted at Princess Margaret Hospital using medication chart records randomly selected from 145,550 patient encounters from the Emergency Department, Inpatient Wards and Outpatient Clinics. Patient and prescribing data were collected. Drugs were classified as off-label or unlicensed based on Australian registration data. A hierarchical system of age, indication, route of administration and dosage was used. Drugs were classified according to the Anatomical Therapeutic Chemical Code.
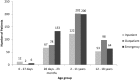
Results: A total of 1,037 paediatric patients were selected where 2,654 prescriptions for 330 different drugs were prescribed to 699 patients (67.4%). Most off-label drugs (n = 295; 43.3%) were from the nervous system; a majority of unlicensed drugs were systemic hormonal preparations excluding sex hormones (n = 22, 32.4%). Inpatients were prescribed more off-label drugs than outpatients or Emergency Department patients (p < 0.0001). Most off-label prescribing occurred in infants and children (31.7% and 35.9% respectively) and the highest percentage of unlicensed prescribing (7.2%) occurred in infants (p < 0.0001). There were 25.7% of off-label and 2.6% of unlicensed medications prescribed across all three settings. Common reasons for off-label prescribing were dosage (47.4%) and age (43.2%).
Conclusion: This study confirmed off-label and unlicensed use of drugs remains common. Further, that prevalence of both is influenced by the clinical setting, which has implications in regards to medication misadventure, and the need to have systems in place to minimise medication errors. Further, there remains a need for changes in the regulatory system in Australia to ensure that manufacturers incorporate, as it becomes available, evidence regarding efficacy and safety of their drugs in children in the official product information.
Conflict of interest statement
Figures
References
-
- O'Donnell CP, Stone R, Morley C (2002) Unlicensed and off-label drug use in an Australian neonatal intensive care unit. Pediatrics 110 (5): e52–e52 - PubMed
-
- Turner S, Gill A, Nunn T, Hewitt B, Choonara I (1996) Use of "off-label" and unlicensed drugs in paediatric intensive care unit. Lancet 347 (9000): 549–550 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources