Differential Susceptibility of Human Pleural and Peritoneal Mesothelial Cells to Asbestos Exposure
- PMID: 25757056
- PMCID: PMC4698803
- DOI: 10.1002/jcb.25095
Differential Susceptibility of Human Pleural and Peritoneal Mesothelial Cells to Asbestos Exposure
Abstract
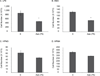
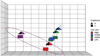
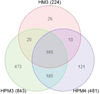
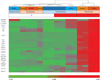
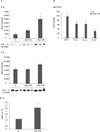
Malignant mesothelioma (MM) is an aggressive cancer of mesothelial cells of pleural and peritoneal cavities. In 85% of cases both pleural and peritoneal MM is caused by asbestos exposure. Although both are asbestos-induced cancers, the incidence of pleural MM is significantly higher (85%) than peritoneal MM (15%). It has been proposed that carcinogenesis is a result of asbestos-induced inflammation but it is not clear what contributes to the differences observed between incidences of these two cancers. We hypothesize that the observed differences in incidences of pleural and peritoneal MM are the result of differences in the direct response of these cell types to asbestos rather than to differences mediated by the in vivo microenvironment. To test this hypothesis we characterized cellular responses to asbestos in a controlled environment. We found significantly greater changes in genome-wide expression in response to asbestos exposure in pleural mesothelial cells as compared to peritoneal mesothelial cells. In particular, a greater response in many common genes (IL-8, ATF3, CXCL2, CXCL3, IL-6, GOS2) was seen in pleural mesothelial cells as compared to peritoneal mesothelial cells. Unique genes expressed in pleural mesothelial cells were mainly pro-inflammatory (G-CSF, IL-1β, IL-1α, GREM1) and have previously been shown to be involved in development of MM. Our results are consistent with the hypothesis that differences in incidences of pleural and peritoneal MM upon exposure to asbestos are the result of differences in mesothelial cell physiology that lead to differences in the inflammatory response, which leads to cancer.
Keywords: ASBESTOS; INFLAMMATION; MASSIVELY PARALLEL SEQUENCING; MESOTHELIAL CELLS; MESOTHELIOMA; RNA-SEQ.
© 2015 Wiley Periodicals, Inc.
Figures



References
-
- Adachi Y, Aoki C, et al. Interleukin-6 induces both cell growth and VEGF production in malignant mesotheliomas. Int J Cancer. 2006;119(6):1303–1311. - PubMed
-
- Adachi Y, Yoshio-Hoshino N, et al. VEGF targeting in mesotheliomas using an interleukin-6 signal inhibitor based on adenovirus gene delivery. Anticancer Res. 2010;30(6):1947–1952. - PubMed
-
- Amati M, Tomasetti M, et al. Assessment of biomarkers in asbestos-exposed workers as indicators of cancer risk. Mutat Res. 2008;655(1–2):52–58. - PubMed
-
- Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecology. 2001;26:32–46.
-
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. 2009;57(1):289–300. Series B (Methodological)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

