The osteogenic response of mesenchymal stromal cells to strontium-substituted bioactive glasses
- PMID: 25757935
- PMCID: PMC5053305
- DOI: 10.1002/term.2003
The osteogenic response of mesenchymal stromal cells to strontium-substituted bioactive glasses
Abstract
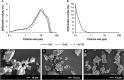
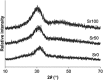
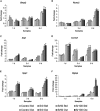
Bioactive glasses are known to stimulate bone healing, and the incorporation of strontium has the potential to increase their potency. In this study, calcium oxide in the 45S5 bioactive glass composition was partially (50%, Sr50) or fully (100%, Sr100) substituted with strontium oxide on a molar basis. The effects of the substitution on bioactive glass properties were studied, including density, solubility, and in vitro cytotoxicity. Stimulation of osteogenic differentiation was investigated using mesenchymal stromal cells obtained from rat bone marrow. Strontium substitution resulted in altered physical properties including increased solubility. Statistically significant reductions in cell viability were observed with the addition of bioactive glass powders to culture medium. Specifically, addition of ≥ 13.3 mg/ml of 45S5 bioactive glass or Sr50, or ≥ 6.7 mg/ml of Sr100, resulted in significant inhibition. Real-time PCR analyses detected the upregulation of genes associated with osteoblastic differentiation in the presence of all bioactive glass compositions. Some genes, including Alpl and Bglap, were further stimulated in the presence of Sr50 and Sr100. It was concluded that strontium-substituted bioactive glasses promoted osteogenesis in a differentiating bone cell culture model and, therefore, have considerable potential for use as improved bioactive glasses for bone tissue regeneration.
Keywords: bioactive glass; mesenchymal stromal cells; osteogenic differentiation; real-time PCR; strontium.
© 2015 The Authors. Journal of Tissue Engineering and Regenerative Medicine Published by John Wiley & Sons Ltd.
Figures



References
-
- Barradas AMC, Fernandes HAM, Groen N et al 2012; A calcium‐induced signaling cascade leading to osteogenic differentiation of human bone marrow‐derived mesenchymal stromal cells. Biomaterials 33: 3205–3215. - PubMed
-
- Bonnelye E, Chabadel A, Saltel F et al 2008; Dual effect of strontium ranelate: stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro . Bone 42: 129–138. - PubMed
-
- Bosetti M, Cannas M. 2005; The effect of bioactive glasses on bone marrow stromal cells differentiation. Biomaterials 26: 3873–3879. - PubMed
-
- Clupper DC, Hench LL. 2003; Crystallization kinetics of tape cast bioactive glass 45S5. J Non‐Cryst Solids 318: 43–48.
-
- Doweidar H. 1996; The density of alkali silicate glasses in relation to the microstructure. J Non‐Cryst Solids 194: 155–162.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

