In vivo CaspaseTracker biosensor system for detecting anastasis and non-apoptotic caspase activity
- PMID: 25757939
- PMCID: PMC4355673
- DOI: 10.1038/srep09015
In vivo CaspaseTracker biosensor system for detecting anastasis and non-apoptotic caspase activity
Abstract
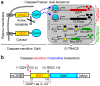
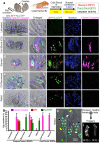
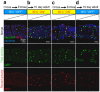
The discovery that mammalian cells can survive late-stage apoptosis challenges the general assumption that active caspases are markers of impending death. However, tools have not been available to track healthy cells that have experienced caspase activity at any time in the past. Therefore, to determine if cells in whole animals can undergo reversal of apoptosis, known as anastasis, we developed a dual color CaspaseTracker system for Drosophila to identify cells with ongoing or past caspase activity. Transient exposure of healthy females to environmental stresses such as cold shock or starvation activated the CaspaseTracker coincident with caspase activity and apoptotic morphologies in multiple cell types of developing egg chambers. Importantly, when stressed flies were returned to normal conditions, morphologically healthy egg chambers and new progeny flies were labeled by the biosensor, suggesting functional recovery from apoptotic caspase activation. In striking contrast to developing egg chambers, which lack basal caspase biosensor activation under normal conditions, many adult tissues of normal healthy flies exhibit robust caspase biosensor activity in a portion of cells, including neurons. The widespread persistence of CaspaseTracker-positivity implies that healthy cells utilize active caspases for non-apoptotic physiological functions during and after normal development.
Figures

References
-
- Alnemri E. S. et al. Human ICE/CED-3 protease nomenclature. Cell 87, 171 (1996). - PubMed
-
- Riedl S. J. & Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol 5, 897–907 (2004). - PubMed
-
- Chai J. & Shi Y. Apoptosome and inflammasome: conserved machineries for caspase activation. National Science Review 1, 101–118 (2013).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

