Prevention of exercised induced cardiomyopathy following Pip-PMO treatment in dystrophic mdx mice
- PMID: 25758104
- PMCID: PMC4355666
- DOI: 10.1038/srep08986
Prevention of exercised induced cardiomyopathy following Pip-PMO treatment in dystrophic mdx mice
Abstract
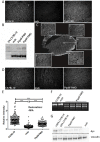
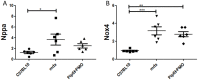
Duchenne muscular dystrophy (DMD) is a fatal neuromuscular disorder caused by mutations in the Dmd gene. In addition to skeletal muscle wasting, DMD patients develop cardiomyopathy, which significantly contributes to mortality. Antisense oligonucleotides (AOs) are a promising DMD therapy, restoring functional dystrophin protein by exon skipping. However, a major limitation with current AOs is the absence of dystrophin correction in heart. Pip peptide-AOs demonstrate high activity in cardiac muscle. To determine their therapeutic value, dystrophic mdx mice were subject to forced exercise to model the DMD cardiac phenotype. Repeated peptide-AO treatments resulted in high levels of cardiac dystrophin protein, which prevented the exercised induced progression of cardiomyopathy, normalising heart size as well as stabilising other cardiac parameters. Treated mice also exhibited significantly reduced cardiac fibrosis and improved sarcolemmal integrity. This work demonstrates that high levels of cardiac dystrophin restored by Pip peptide-AOs prevents further deterioration of cardiomyopathy and pathology following exercise in dystrophic DMD mice.
Conflict of interest statement
A.F.S. and M.J.G. (MRC Technology) and C.A.B., S.M.H. and M.J.A.W. (University of Oxford) are named contributors and benefactors of a patent filed for Pip-PMO technologies described herein. C.A.C., A.M.L.C.S., C.G., G.M., M.A.V., T.C.R. and K.C. declare no competing financial interests
Figures


References
-
- Emery A. E. Population frequencies of inherited neuromuscular diseases--a world survey. Neuromuscul Disord 1, 19–29 (1991). - PubMed
-
- Kirchmann C., Kececioglu D., Korinthenberg R. & Dittrich S. Echocardiographic and electrocardiographic findings of cardiomyopathy in Duchenne and Becker-Kiener muscular dystrophies. Pediatric cardiology 26, 66–72 (2005). - PubMed
-
- Nigro G., Comi L. I., Politano L. & Bain R. J. The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. Int J Cardiol 26, 271–277 (1990). - PubMed
-
- Bushby K., Muntoni F. & Bourke J. P. 107th ENMC international workshop: the management of cardiac involvement in muscular dystrophy and myotonic dystrophy. 7th–9th June 2002, Naarden, the Netherlands. Neuromuscul Disord 13, 166–172 (2003). - PubMed
-
- Sultan A. & Fayaz M. Prevalence of cardiomyopathy in Duchenne and Becker's muscular dystrophy. Journal of Ayub Medical College, Abbottabad: JAMC 20, 7–13 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

