Lipofuscin redistribution and loss accompanied by cytoskeletal stress in retinal pigment epithelium of eyes with age-related macular degeneration
- PMID: 25758814
- PMCID: PMC4455310
- DOI: 10.1167/iovs.14-16274
Lipofuscin redistribution and loss accompanied by cytoskeletal stress in retinal pigment epithelium of eyes with age-related macular degeneration
Abstract
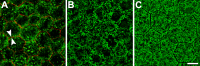
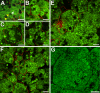
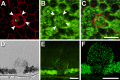
Purpose: Lipofuscin (LF) and melanolipofuscin (MLF) of the retinal pigment epithelium (RPE) are the principal sources of autofluorescence (AF) signals in clinical fundus-AF imaging. Few details about the subcellular distribution of AF organelles in AMD are available. We describe the impact of aging and AMD on RPE morphology revealed by the distribution of AF LF/MLF granules and actin cytoskeleton in human tissues.
Methods: Thirty-five RPE-Bruch's membrane flatmounts from 35 donors were prepared (postmortem: ≤4 hours). Ex vivo fundus examination at the time of accession revealed either absence of chorioretinal pathologies (10 tissues; mean age: 83.0 ± 2.6 years) or stages of AMD (25 tissues; 85.0 ± 5.8 years): early AMD, geographic atrophy, and late exudative AMD. Retinal pigment epithelium cytoskeleton was labeled with AlexaFluor647-Phalloidin. Tissues were imaged on a spinning-disk fluorescence microscope and a high-resolution structured illumination microscope.
Results: Age-related macular degeneration impacts individual RPE cells by (1) lipofuscin redistribution by (i) degranulation (granule-by-granule loss) and/or (ii) aggregation and apparent shedding into the extracellular space; (2) enlarged RPE cell area and conversion from convex to irregular and sometimes concave polygons; and (3) cytoskeleton derangement including separations and breaks around subretinal deposits, thickening, and stress fibers.
Conclusions: We report an extensive and systematic en face analysis of LF/MLF-AF in AMD eyes. Redistribution and loss of AF granules are among the earliest AMD changes and could reduce fundus AF signal attributable to RPE at these locations. Data can enhance the interpretation of clinical fundus-AF and provide a basis for future quantitative studies.
Figures



References
-
- Delori FC,, Dorey CK,, Staurenghi G,, Arend O,, Goger DG,, Weiter JJ. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest Ophthalmol Vis Sci. 1995; 36: 718–729. - PubMed
-
- Schmitz-Valckenberg S,, Fleckenstein M,, Scholl HP,, Holz FG. Fundus autofluorescence and progression of age-related macular degeneration. Surv Ophthalmol. 2009; 54: 96–117. - PubMed
-
- von Ruckmann A,, Fitzke FW,, Bird AC. Fundus autofluorescence in age-related macular disease imaged with a laser scanning ophthalmoscope. Invest Ophthalmol Vis Sci. 1997; 38: 478–486. - PubMed
-
- Delori FC,, Fleckner MR,, Goger DG,, Weiter JJ,, Dorey CK. Autofluorescence distribution associated with drusen in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2000; 41: 496–504. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

