Paraoxon and Pyridostigmine Interfere with Neural Stem Cell Differentiation
- PMID: 25758980
- PMCID: PMC4567516
- DOI: 10.1007/s11064-015-1548-7
Paraoxon and Pyridostigmine Interfere with Neural Stem Cell Differentiation
Abstract
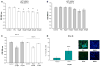
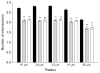
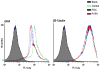
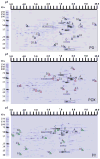
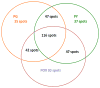
Acetylcholinesterase (AChE) inhibition has been described as the main mechanism of organophosphate (OP)-evoked toxicity. OPs represent a human health threat, because chronic exposure to low doses can damage the developing brain, and acute exposure can produce long-lasting damage to adult brains, despite post-exposure medical countermeasures. Although the main mechanism of OP toxicity is AChE inhibition, several lines of evidence suggest that OPs also act by other mechanisms. We hypothesized that rat neural progenitor cells extracted on embryonic day 14.5 would be affected by constant inhibition of AChE from chronic exposure to OP or pyridostigmine (a reversible AChE blocker) during differentiation. In this work, the OP paraoxon decreased cell viability in concentrations >50 μM, as measured with the MTT assay; however, this effect was not dose-dependent. Reduced viability could not be attributed to blockade of AChE activity, since treatment with 200 µM pyridostigmine did not affect cell viability, even after 6 days. Although changes in protein expression patterns were noted in both treatments, the distribution of differentiated phenotypes, such as the percentages of neurons and glial cells, was not altered, as determined by flow cytometry. Since paraoxon and pyridostigmine each decreased neurite outgrowth (but did not prevent differentiation), we infer that developmental patterns may have been affected.
Keywords: Differentiation; Neural progenitor cells; Organophosphates; Paraoxon; Proteomics.
Conflict of interest statement
Figures
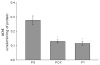

References
-
- Casida JE, Quistad GB. Organophosphate toxicology: safety aspects of nonacetylcholinesterase secondary targets. Chem Res Toxicol. 2004;17(8):983–998. - PubMed
-
- Hail SL, Obafemi A, Kleinschmidt KC. Successful management of olanzapine-induced anticholinergic agitation and delirium with a continuous intravenous infusion of physostigmine in a pediatric patient. Clin Toxicol (Phila) 2013;51(3):162–166. - PubMed
-
- Butler AM, Murray M. Biotransformation of parathion in human liver: participation of CYP3A4 and its inactivation during mi-crosomal parathion oxidation. J Pharmacol Exp Ther. 1997;280(2):966–973. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

