Pharmacokinetics of anti-TB drugs in Malawian children: reconsidering the role of ethambutol
- PMID: 25759035
- PMCID: PMC4498297
- DOI: 10.1093/jac/dkv039
Pharmacokinetics of anti-TB drugs in Malawian children: reconsidering the role of ethambutol
Abstract
Background: Current guidelines for dosing of anti-TB drugs in children advocate higher doses for rifampicin and isoniazid despite limited availability of paediatric data on the pharmacokinetics of these drugs, especially from Africa, where the burden of childhood disease remains high.
Methods: Thirty children aged 6 months to 15 years underwent intensive pharmacokinetic sampling for first-line anti-TB drugs at Queen Elizabeth Central Hospital, Blantyre, Malawi. Rifampicin, isoniazid, pyrazinamide and ethambutol were dosed at 10, 5, 25 and 20 mg/kg, respectively. Plasma drug concentrations were determined using sensitive, validated bioanalytical methods and summary pharmacokinetic parameters were estimated using non-compartmental analysis.
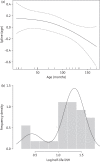
Results: The median (IQR) Cmax was 2.90 (2.08-3.43), 3.37 (2.55-4.59), 34.60 (32.30-40.90) and 1.20 (0.85-1.68) mg/L while the median (IQR) AUC0-∞ was 16.92 (11.10-22.74), 11.48 (7.35-18.93), 333.50 (279.50-487.2) and 8.65 (5.96-11.47) mg·h/L for rifampicin, isoniazid, pyrazinamide and ethambutol, respectively. For all drugs, pharmacokinetic parameters relating to drug absorption and exposure were lower than those published for adults, though similar to existing paediatric data from sub-Saharan Africa. Weight and/or dose predicted at least one measure of exposure for all drugs. Age-related decreases in CL/F for rifampicin and pyrazinamide and a biphasic elimination pattern of isoniazid were observed. Predicted AUC0 -∞ for rifampicin dosed at 15 mg/kg was comparable to that of adults while the dose required to achieve ethambutol exposure similar to that in adults was 55 mg/kg or higher.
Conclusions: These data support recently revised WHO recommendations for dosing of anti-TB drugs in children, but dosing of ethambutol in children also appears inadequate by comparison with adult pharmacokinetic data.
Keywords: Africa; PK; paediatrics; tuberculosis.
© The Author 2015. Published by Oxford University Press on behalf of the British Society for Antimicrobial Chemotherapy.
Figures


References
-
- Donald PR, Ahmed A, Burman WJ, et al. Requirements for the clinical evaluation of new anti-tuberculosis agents in children. Int J Tuberc Lung Dis 2013; 17: 794–9. - PubMed
-
- Donald PR, Maritz JS, Diacon AH. Pyrazinamide pharmacokinetics and efficacy in adults and children. Tuberculosis (Edinb) 2012; 92: 1–8. - PubMed
-
- Donald PR, Maritz JS, Diacon AH. The pharmacokinetics and pharmacodynamics of rifampicin in adults and children in relation to the dosage recommended for children. Tuberculosis (Edinb) 2011; 91: 196–207. - PubMed
-
- Donald PR, Maher D, Maritz JS, et al. Ethambutol dosage for the treatment of children: literature review and recommendations. Int J Tuberc Lung Dis 2006; 10: 1318–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

