Three-dimensional architecture of podocytes revealed by block-face scanning electron microscopy
- PMID: 25759085
- PMCID: PMC4355681
- DOI: 10.1038/srep08993
Three-dimensional architecture of podocytes revealed by block-face scanning electron microscopy
Abstract
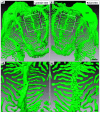
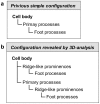
Block-face imaging is a scanning electron microscopic technique which enables easier acquisition of serial ultrastructural images directly from the surface of resin-embedded biological samples with a similar quality to transmission electron micrographs. In the present study, we analyzed the three-dimensional architecture of podocytes using serial block-face imaging. It was previously believed that podocytes are divided into three kinds of subcellular compartment: cell body, primary process, and foot process, which are simply aligned in this order. When the reconstructed podocytes were viewed from their basal side, the foot processes were branched from a ridge-like prominence, which was formed on the basal surface of the primary process and was similar to the usual foot processes in structure. Moreover, from the cell body, the foot processes were also emerged via the ridge-like prominence, as found in the primary process. The ridge-like prominence anchored the cell body and primary process to the glomerular basement membrane, and connected the foot processes to the cell body and primary process. In conclusion, serial block-face imaging is a powerful tool for clear understanding the three-dimensional architecture of podocytes through its ability to reveal novel structures which were difficult to determine by conventional transmission and scanning electron microscopes alone.
Figures





References
-
- Kriz W. & Kaissling B. Structural organization of the mammalian kidney. In:: The Kidney, Physiology and Pathophysiology (eds Seldin, D. W. & Giebisch, G.). 3rd edn. Lippincott Williams & Wilkins (2000).
-
- Andrews P. M. & Porter K. R. A scanning electron microscopic study of the nephron. Am J Anat 140, 81–115 (1974). - PubMed
-
- Fujita T., Tokunaga J. & Miyoshi M. Scanning electron microscopy of the podocytes of renal glomerulus. Arch Histol Jpn 32, 99–113 (1970). - PubMed
-
- Karlsson U. Three-dimensional studies of neurons in the lateral geniculate nucleus of the rat. I. Organelle organization in the perikaryon and its proximal branches. J Ultrastruct Res 16, 429–481 (1966). - PubMed
-
- Hoffpauir B. K., Pope B. A. & Spirou G. A. Serial sectioning and electron microscopy of large tissue volumes for 3D analysis and reconstruction: a case study of the calyx of Held. Nat Protoc 2, 9–22 (2007). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

