Current ADC Linker Chemistry
- PMID: 25759187
- PMCID: PMC4596905
- DOI: 10.1007/s11095-015-1657-7
Current ADC Linker Chemistry
Abstract
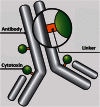
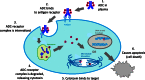
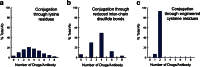
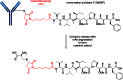
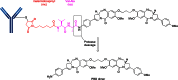
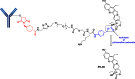
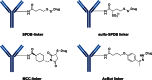
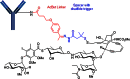
The list of ADCs in the clinic continues to grow, bolstered by the success of first two marketed ADCs: ADCETRIS® and Kadcyla®. Currently, there are 40 ADCs in various phases of clinical development. However, only 34 of these have published their structures. Of the 34 disclosed structures, 24 of them use a linkage to the thiol of cysteines on the monoclonal antibody. The remaining 10 candidates utilize chemistry to surface lysines of the antibody. Due to the inherent heterogeneity of conjugation to the multiple lysines or cysteines found in mAbs, significant research efforts are now being directed toward the production of discrete, homogeneous ADC products, via site-specific conjugation. These site-specific conjugations may involve genetic engineering of the mAb to introduce discrete, available cysteines or non-natural amino acids with an orthogonally-reactive functional group handle such as an aldehyde, ketone, azido, or alkynyl tag. These site-specific approaches not only increase the homogeneity of ADCs but also enable novel bio-orthogonal chemistries that utilize reactive moieties other than thiol or amine. This broadens the diversity of linkers that can be utilized which will lead to better linker design in future generations of ADCs.
Keywords: ADC; ADC clinical candidates; antibody-drug conjugates; bioconjugates; linker chemistry.
Figures







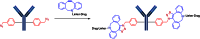
References
-
- Trail PA. Antibody drug conjugates as cancer therapeutics. Antibodies. 2013;2:113–29. doi: 10.3390/antib2010113. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

