Secretome analysis of Vibrio cholerae type VI secretion system reveals a new effector-immunity pair
- PMID: 25759499
- PMCID: PMC4453574
- DOI: 10.1128/mBio.00075-15
Secretome analysis of Vibrio cholerae type VI secretion system reveals a new effector-immunity pair
Abstract
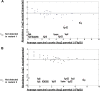
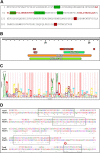
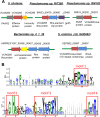
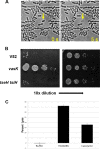
The type VI secretion system (T6SS) is a dynamic macromolecular organelle that many Gram-negative bacteria use to inhibit or kill other prokaryotic or eukaryotic cells. The toxic effectors of T6SS are delivered to the prey cells in a contact-dependent manner. In Vibrio cholerae, the etiologic agent of cholera, T6SS is active during intestinal infection. Here, we describe the use of comparative proteomics coupled with bioinformatics to identify a new T6SS effector-immunity pair. This analysis was able to identify all previously identified secreted substrates of T6SS except PAAR (proline, alanine, alanine, arginine) motif-containing proteins. Additionally, this approach led to the identification of a new secreted protein encoded by VCA0285 (TseH) that carries a predicted hydrolase domain. We confirmed that TseH is toxic when expressed in the periplasm of Escherichia coli and V. cholerae cells. The toxicity observed in V. cholerae was suppressed by coexpression of the protein encoded by VCA0286 (TsiH), indicating that this protein is the cognate immunity protein of TseH. Furthermore, exogenous addition of purified recombinant TseH to permeabilized E. coli cells caused cell lysis. Bioinformatics analysis of the TseH protein sequence suggest that it is a member of a new family of cell wall-degrading enzymes that include proteins belonging to the YD repeat and Rhs superfamilies and that orthologs of TseH are likely expressed by species belonging to phyla as diverse as Bacteroidetes and Proteobacteria.
Importance: The Gram-negative bacterium Vibrio cholerae causes cholera, a severe and often lethal diarrheal disease. The 2010-2012 epidemic in Haiti and new explosive epidemics in Africa show that cholera remains a significant global public health problem. The type VI secretion system (T6SS) is a dynamic organelle expressed by many Gram-negative bacteria, which use it to inject toxic effector proteins into eukaryotic and bacterial prey cells. In this study, we applied a comparative proteomics approach to the V. cholerae T6SS secretome to identify new substrates of this secretion apparatus. We show that the product of the gene VCA0285 is likely a new peptidoglycan hydrolase that is secreted by T6SS and that its cognate immunity protein is encoded by the gene that is immediately downstream (VCA0286). Bioinformatics analysis shows that VCA0285 carries four conserved motifs that likely define a large family of hydrolases with antibacterial activity. The identification of new antibacterial T6SS effectors provides useful information for the development of novel antibiotics and therapeutic agents.
Copyright © 2015 Altindis et al.
Figures

References
-
- Pukatzki S, Ma AT, Sturtevant D, Krastins B, Sarracino D, Nelson WC, Heidelberg JF, Mekalanos JJ. 2006. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc Natl Acad Sci U S A 103:1528–1533. doi: 10.1073/pnas.0510322103. - DOI - PMC - PubMed
-
- Leiman PG, Basler M, Ramagopal UA, Bonanno JB, Sauder JM, Pukatzki S, Burley SK, Almo SC, Mekalanos JJ. 2009. Type VI secretion apparatus and phage tail-associated protein complexes share a common evolutionary origin. Proc Natl Acad Sci U S A 106:4154–4159. doi: 10.1073/pnas.0813360106. - DOI - PMC - PubMed
-
- Russell AB, Wexler AG, Harding BN, Whitney JC, Bohn AJ, Goo YA, Tran BQ, Barry NA, Zheng H, Peterson SB, Chou S, Gonen T, Goodlett DR, Goodman AL, Mougous JD. 2014. A type VI secretion-related pathway in Bacteroidetes mediates interbacterial antagonism. Cell Host Microbe 16:227–236. doi: 10.1016/j.chom.2014.07.007. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

