Vaccination with adjuvanted recombinant neuraminidase induces broad heterologous, but not heterosubtypic, cross-protection against influenza virus infection in mice
- PMID: 25759506
- PMCID: PMC4453582
- DOI: 10.1128/mBio.02556-14
Vaccination with adjuvanted recombinant neuraminidase induces broad heterologous, but not heterosubtypic, cross-protection against influenza virus infection in mice
Abstract
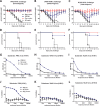
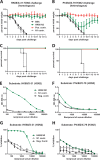
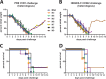
In an attempt to assess the cross-protective potential of the influenza virus neuraminidase (NA) as a vaccine antigen, different subtypes of recombinant NA were expressed in a baculovirus system and used to vaccinate mice prior to lethal challenge with homologous, heterologous, or heterosubtypic viruses. Mice immunized with NA of subtype N2 were completely protected from morbidity and mortality in a homologous challenge and displayed significantly reduced viral lung titers. Heterologous challenge with a drifted strain resulted in morbidity but no mortality. Similar results were obtained for challenge experiments with N1 NA. Mice immunized with influenza B virus NA (from B/Yamagata/16/88) displayed no morbidity when sublethally infected with the homologous strain and, importantly, were completely protected from morbidity and mortality when lethally challenged with the prototype Victoria lineage strain or a more recent Victoria lineage isolate. Upon analyzing the NA content in 4 different inactivated-virus vaccine formulations from the 2013-2014 season via Western blot assay and enzyme-linked immunosorbent assay quantification, we found that the amount of NA does indeed vary across vaccine brands. We also measured hemagglutinin (HA) and NA endpoint titers in pre- and postvaccination human serum samples from individuals who received a trivalent inactivated seasonal influenza vaccine from the 2004-2005 season; the induction of NA titers was statistically less pronounced than the induction of HA titers. The demonstrated homologous and heterologous protective capacity of recombinant NA suggests that supplementing vaccine formulations with a standard amount of NA may offer increased protection against influenza virus infection.
Importance: Despite the existence of vaccine prophylaxis and antiviral therapeutics, the influenza virus continues to cause morbidity and mortality in the human population, emphasizing the continued need for research in the field. While the majority of influenza vaccine strategies target the viral hemagglutinin, the immunodominant antigen on the surface of the influenza virion, antibodies against the viral neuraminidase (NA) have been correlated with less severe disease and decreased viral shedding in humans. Nevertheless, the amount of NA is not standardized in current seasonal vaccines, and the exact breadth of NA-based protection is unknown. Greater insight into the cross-protective potential of influenza virus NA as a vaccine antigen may pave the way for the development of influenza vaccines of greater breadth and efficacy.
Copyright © 2015 Wohlbold et al.
Figures

Similar articles
-
Vaccination with Recombinant Parainfluenza Virus 5 Expressing Neuraminidase Protects against Homologous and Heterologous Influenza Virus Challenge.J Virol. 2017 Nov 14;91(23):e01579-17. doi: 10.1128/JVI.01579-17. Print 2017 Dec 1. J Virol. 2017. PMID: 28931689 Free PMC article.
-
Neuraminidase-Inhibiting Antibody Titers Correlate with Protection from Heterologous Influenza Virus Strains of the Same Neuraminidase Subtype.J Virol. 2018 Aug 16;92(17):e01006-18. doi: 10.1128/JVI.01006-18. Print 2018 Sep 1. J Virol. 2018. PMID: 29925654 Free PMC article.
-
Mucosal administration of raccoonpox virus expressing highly pathogenic avian H5N1 influenza neuraminidase is highly protective against H5N1 and seasonal influenza virus challenge.Vaccine. 2015 Sep 22;33(39):5155-62. doi: 10.1016/j.vaccine.2015.08.005. Epub 2015 Aug 10. Vaccine. 2015. PMID: 26271828
-
Influenza Neuraminidase Characteristics and Potential as a Vaccine Target.Front Immunol. 2021 Nov 16;12:786617. doi: 10.3389/fimmu.2021.786617. eCollection 2021. Front Immunol. 2021. PMID: 34868073 Free PMC article. Review.
-
In the shadow of hemagglutinin: a growing interest in influenza viral neuraminidase and its role as a vaccine antigen.Viruses. 2014 Jun 23;6(6):2465-94. doi: 10.3390/v6062465. Viruses. 2014. PMID: 24960271 Free PMC article. Review.
Cited by
-
Impact of Individual Viral Gene Segments from Influenza A/H5N8 Virus on the Protective Efficacy of Inactivated Subtype-Specific Influenza Vaccine.Pathogens. 2021 Mar 19;10(3):368. doi: 10.3390/pathogens10030368. Pathogens. 2021. PMID: 33808583 Free PMC article.
-
Immunity induced by vaccination with recombinant influenza B virus neuraminidase protein breaks viral transmission chains in guinea pigs in an exposure intensity-dependent manner.bioRxiv [Preprint]. 2022 Oct 20:2022.10.19.512980. doi: 10.1101/2022.10.19.512980. bioRxiv. 2022. Update in: J Virol. 2023 Oct 31;97(10):e0105723. doi: 10.1128/jvi.01057-23. PMID: 36299418 Free PMC article. Updated. Preprint.
-
Immunity to Influenza Infection in Humans.Cold Spring Harb Perspect Med. 2021 Mar 1;11(3):a038729. doi: 10.1101/cshperspect.a038729. Cold Spring Harb Perspect Med. 2021. PMID: 31871226 Free PMC article. Review.
-
Monoclonal Antibodies with Neutralizing Activity and Fc-Effector Functions against the Machupo Virus Glycoprotein.J Virol. 2020 Feb 14;94(5):e01741-19. doi: 10.1128/JVI.01741-19. Print 2020 Feb 14. J Virol. 2020. PMID: 31801871 Free PMC article.
-
Repeated Influenza Vaccination Boosts and Maintains H1N1pdm09 Neuraminidase Antibody Titers.Front Immunol. 2021 Oct 14;12:748264. doi: 10.3389/fimmu.2021.748264. eCollection 2021. Front Immunol. 2021. PMID: 34721417 Free PMC article.
References
-
- De Jong JC, Beyer WE, Palache AM, Rimmelzwaan GF, Osterhaus AD. 2000. Mismatch between the 1997/1998 influenza vaccine and the major epidemic A(H3N2) virus strain as the cause of an inadequate vaccine-induced antibody response to this strain in the elderly. J Med Virol 61:94–99. doi:10.1002/(SICI)1096-9071(200005)61:1<94::AID-JMV15>3.0.CO;2-C. - DOI - PubMed
-
- Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, James J, Air GM, Capra JD, Ahmed R, Wilson PC. 2008. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 453:667–671. doi:10.1038/nature06890. - DOI - PMC - PubMed
-
- Moody MA, Zhang R, Walter EB, Woods CW, Ginsburg GS, McClain MT, Denny TN, Chen X, Munshaw S, Marshall DJ, Whitesides JF, Drinker MS, Amos JD, Gurley TC, Eudailey JA, Foulger A, DeRosa KR, Parks R, Meyerhoff RR, Yu JS, Kozink DM, Barefoot BE, Ramsburg EA, Khurana S, Golding H, Vandergrift NA, Alam SM, Tomaras GD, Kepler TB, Kelsoe G, Liao HX, Haynes BF. 2011. H3N2 influenza infection elicits more cross-reactive and less clonally expanded anti-hemagglutinin antibodies than influenza vaccination. PLoS One 6:e25797. doi:10.1371/journal.pone.0025797. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
