Koenimbin, a natural dietary compound of Murraya koenigii (L) Spreng: inhibition of MCF7 breast cancer cells and targeting of derived MCF7 breast cancer stem cells (CD44(+)/CD24(-/low)): an in vitro study
- PMID: 25759564
- PMCID: PMC4346015
- DOI: 10.2147/DDDT.S72127
Koenimbin, a natural dietary compound of Murraya koenigii (L) Spreng: inhibition of MCF7 breast cancer cells and targeting of derived MCF7 breast cancer stem cells (CD44(+)/CD24(-/low)): an in vitro study
Abstract
Background: Inhibition of breast cancer stem cells has been shown to be an effective therapeutic strategy for cancer prevention. The aims of this work were to evaluate the efficacy of koenimbin, isolated from Murraya koenigii (L) Spreng, in the inhibition of MCF7 breast cancer cells and to target MCF7 breast cancer stem cells through apoptosis in vitro.
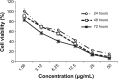
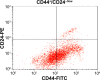
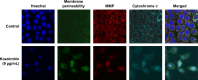
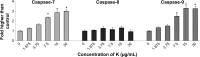
Methods: Koenimbin-induced cell viability was evaluated using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. Nuclear condensation, cell permeability, mitochondrial membrane potential, and cytochrome c release were observed using high-content screening. Cell cycle arrest was examined using flow cytometry, while human apoptosis proteome profiler assays were used to investigate the mechanism of apoptosis. Protein expression levels of Bax, Bcl2, and heat shock protein 70 were confirmed using Western blotting. Caspase-7, caspase-8, and caspase-9 levels were measured, and nuclear factor kappa B (NF-κB) activity was assessed using a high-content screening assay. Aldefluor™ and mammosphere formation assays were used to evaluate the effect of koenimbin on MCF7 breast cancer stem cells in vitro. The Wnt/β-catenin signaling pathway was investigated using Western blotting.
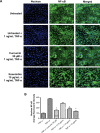
Results: Koenimbin-induced apoptosis in MCF7 cells was mediated by cell death-transducing signals regulating the mitochondrial membrane potential by downregulating Bcl2 and upregulating Bax, due to cytochrome c release from the mitochondria to the cytosol. Koenimbin induced significant (P<0.05) sub-G0 phase arrest in breast cancer cells. Cytochrome c release triggered caspase-9 activation, which then activated caspase-7, leading to apoptotic changes. This form of apoptosis is closely associated with the intrinsic pathway and inhibition of NF-κB translocation from the cytoplasm to the nucleus. Koenimbin significantly (P<0.05) decreased the aldehyde dehydrogenase-positive cell population in MCF7 cancer stem cells and significantly (P<0.01) decreased the size and number of MCF7 cancer stem cells in primary, secondary, and tertiary mammospheres in vitro. Koenimbin also significantly (P<0.05) downregulated the Wnt/β-catenin self-renewal pathway.
Conclusion: Koenimbin has potential for future chemoprevention studies, and may lead to the discovery of further cancer management strategies by reducing cancer resistance and recurrence and improving patient survival.
Keywords: MCF7 breast cancer stem cells; Murraya koenigii (L) Spreng; Wnt/β-catenin; glycogen synthase kinase 3β; koenimbin; nuclear factor kappa B.
Figures





References
-
- Nakamura S, Nakashima S, Oda Y, et al. Alkaloids from Sri Lankan curry-leaf (Murraya koenigii) display melanogenesis inhibitory activity: structures of karapinchamines A and B. Bioorg Med Chem. 2013;21(5):1043–1049. - PubMed
-
- Ma Q, Tian J, Yang J, et al. Bioactive carbazole alkaloids from Murraya koenigii (L.) Spreng. Fitoterapia. 2013;87:1–6. - PubMed
-
- Arulselvan P, Subramanian S. Effect of Murraya koenigii leaf extract on carbohydrate metabolism studied in streptozotocin induced diabetic rats. Int J Biol Chem. 2007;1(1):21–28.
-
- Vinuthan M, Kumar VG, Narayanaswamy N, Veena T. Lipid lowering effect of aqueous leaves extract of Murraya koenigii (curry leaf) on alloxan-induced male diabetic rats. Pharmacogn Mag. 2007;3(10):112.
-
- Arulselvan P, Subramanian SP. Beneficial effects of Murraya koenigii leaves on antioxidant defense system and ultra structural changes of pancreatic β-cells in experimental diabetes in rats. Chem Biol Interact. 2007;165(2):155–164. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

