Silica nanoparticles induce oxidative stress, inflammation, and endothelial dysfunction in vitro via activation of the MAPK/Nrf2 pathway and nuclear factor-κB signaling
- PMID: 25759575
- PMCID: PMC4345992
- DOI: 10.2147/IJN.S76114
Silica nanoparticles induce oxidative stress, inflammation, and endothelial dysfunction in vitro via activation of the MAPK/Nrf2 pathway and nuclear factor-κB signaling
Abstract
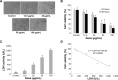
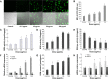
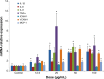
Despite the widespread application of silica nanoparticles (SiNPs) in industrial, commercial, and biomedical fields, their response to human cells has not been fully elucidated. Overall, little is known about the toxicological effects of SiNPs on the cardiovascular system. In this study, SiNPs with a 58 nm diameter were used to study their interaction with human umbilical vein endothelial cells (HUVECs). Dose- and time-dependent decrease in cell viability and damage on cell plasma-membrane integrity showed the cytotoxic potential of the SiNPs. SiNPs were found to induce oxidative stress, as evidenced by the significant elevation of reactive oxygen species generation and malondialdehyde production and downregulated activity in glutathione peroxidase. SiNPs also stimulated release of cytoprotective nitric oxide (NO) and upregulated inducible nitric oxide synthase (NOS) messenger ribonucleic acid, while downregulating endothelial NOS and ET-1 messenger ribonucleic acid, suggesting that SiNPs disturbed the NO/NOS system. SiNP-induced oxidative stress and NO/NOS imbalance resulted in endothelial dysfunction. SiNPs induced inflammation characterized by the upregulation of key inflammatory mediators, including IL-1β, IL-6, IL-8, TNFα, ICAM-1, VCAM-1, and MCP-1. In addition, SiNPs triggered the activation of the Nrf2-mediated antioxidant system, as evidenced by the induction of nuclear factor-κB and MAPK pathway activation. Our findings demonstrated that SiNPs could induce oxidative stress, inflammation, and NO/NOS system imbalance, and eventually lead to endothelial dysfunction via activation of the MAPK/Nrf2 pathway and nuclear factor-κB signaling. This study indicated a potential deleterious effect of SiNPs on the vascular endothelium, which warrants more careful assessment of SiNPs before their application.
Keywords: MAPK; NF-κB; Nrf2; endothelium; oxidative stress; silica nanoparticle.
Figures



Similar articles
-
Amorphous silica nanoparticles trigger vascular endothelial cell injury through apoptosis and autophagy via reactive oxygen species-mediated MAPK/Bcl-2 and PI3K/Akt/mTOR signaling.Int J Nanomedicine. 2016 Oct 11;11:5257-5276. doi: 10.2147/IJN.S112030. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27785026 Free PMC article.
-
Silica nanomaterials induce organ injuries by Ca2+-ROS-initiated disruption of the endothelial barrier and triggering intravascular coagulation.Part Fibre Toxicol. 2020 Mar 23;17(1):12. doi: 10.1186/s12989-020-00340-8. Part Fibre Toxicol. 2020. PMID: 32293491 Free PMC article.
-
Mitochondrial dysfunction, perturbations of mitochondrial dynamics and biogenesis involved in endothelial injury induced by silica nanoparticles.Environ Pollut. 2018 May;236:926-936. doi: 10.1016/j.envpol.2017.10.060. Epub 2017 Nov 1. Environ Pollut. 2018. PMID: 29074197
-
The toxicity of silica nanoparticles to the immune system.Nanomedicine (Lond). 2018 Aug 1;13(15):1939-1962. doi: 10.2217/nnm-2018-0076. Epub 2018 Aug 28. Nanomedicine (Lond). 2018. PMID: 30152253 Review.
-
Toxicology of silica nanoparticles: an update.Arch Toxicol. 2017 Sep;91(9):2967-3010. doi: 10.1007/s00204-017-1993-y. Epub 2017 Jun 1. Arch Toxicol. 2017. PMID: 28573455 Free PMC article. Review.
Cited by
-
Hepatic Cellular Distribution of Silica Nanoparticles by Surface Energy Modification.Int J Mol Sci. 2019 Aug 5;20(15):3812. doi: 10.3390/ijms20153812. Int J Mol Sci. 2019. PMID: 31387201 Free PMC article.
-
Amorphous Silica Nanoparticles Obtained by Laser Ablation Induce Inflammatory Response in Human Lung Fibroblasts.Materials (Basel). 2019 Mar 28;12(7):1026. doi: 10.3390/ma12071026. Materials (Basel). 2019. PMID: 30925685 Free PMC article.
-
In vivo investigation on the chronic hepatotoxicity induced by intraperitoneal administration of 10-nm silicon dioxide nanoparticles.Int J Nanomedicine. 2018 May 7;13:2685-2696. doi: 10.2147/IJN.S162847. eCollection 2018. Int J Nanomedicine. 2018. PMID: 29765215 Free PMC article.
-
Detection of Oxidative Stress Induced by Nanomaterials in Cells-The Roles of Reactive Oxygen Species and Glutathione.Molecules. 2021 Aug 4;26(16):4710. doi: 10.3390/molecules26164710. Molecules. 2021. PMID: 34443297 Free PMC article. Review.
-
The toxicity of SiO2 NPs on cell proliferation and cellular uptake of human lung fibroblastic cell line during the variation of calcination temperature and its modeling by artificial neural network.J Environ Health Sci Eng. 2021 Apr 30;19(1):985-995. doi: 10.1007/s40201-021-00663-4. eCollection 2021 Jun. J Environ Health Sci Eng. 2021. PMID: 34150286 Free PMC article.
References
-
- Bakand S, Hayes A, Dechsakulthorn F. Nanoparticles: a review of particle toxicology following inhalation exposure. Inhal Toxicol. 2012;24(2):125–135. - PubMed
-
- Hansen SF, Michelson ES, Kamper A, Borling P, Stuer-Lauridsen F, Baun A. Categorization framework to aid exposure assessment of nanomaterials in consumer products. Ecotoxicology. 2008;17(5):438–447. - PubMed
-
- Yang Y, Li J. Lipid, protein and poly(NIPAM) coated mesoporous silica nanoparticles for biomedical applications. Adv Colloid Interface Sci. 2014;207:155–163. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

