Layered nanoemulsions as mucoadhesive buccal systems for controlled delivery of oral cancer therapeutics
- PMID: 25759580
- PMCID: PMC4346361
- DOI: 10.2147/IJN.S75474
Layered nanoemulsions as mucoadhesive buccal systems for controlled delivery of oral cancer therapeutics
Abstract
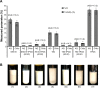
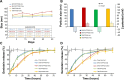
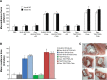
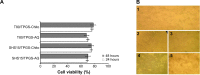
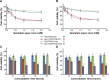
Oral cavity and oropharyngeal cancers are considered the eighth most common cancer worldwide, with relatively poor prognosis (62% of patients surviving 5 years, after diagnosis). The aim of this study was to develop a proof-of-concept mucoadhesive lozenge/buccal tablet, as a potential platform for direct sustained delivery of therapeutic antimitotic nanomedicines. Our system would serve as an adjuvant therapy for oral cancer patients undergoing full-scale diagnostic and operative treatment plans. We utilized lipid-based nanocarriers, namely nanoemulsions (NEs), containing mixed-polyethoxylated emulsifiers and a tocopheryl moiety-enriched oil phase. Prototype NEs, loaded with the proapoptotic lipophilic drug genistein (Gen), were further processed into buccal tablet formulations. The chitosan polyelectrolyte solution overcoat rendered NE droplets cationic, by acting as a mucoadhesive interfacial NE layer. With approximate size of 110 nm, the positively charged chitosan-layered NE (+25 mV) vs negatively charged chitosan-free/primary aqueous NE (-28 mV) exhibited a controlled-release profile and effective mucoadhesion for liquid oral spray prototypes. When punch-pressed, porous NE-based buccal tablets were physically evaluated for hardness, friability, and swelling in addition to ex vivo tissue mucoadhesion force and retention time measurements. Chitosan-containing NE tablets were found equivalent to primary NE and placebo tablets in compression tests, yet significantly superior in all ex vivo adhesion and in vitro release assays (P≤0.05). Following biocompatibility screening of prototype chitosan-layered NEs, substantial anticancer activity of selected cationic Gen-loaded NE formulations, against two oropahryngeal carcinomas, was observed. The data strongly indicate the potential of such nanomucoadhesive systems as maintenance therapy for oral cancer patients awaiting surgical removal, or postresection of identified cancerous lesions.
Keywords: chitosan; genistein; isoflavone; squamous cell carcinomas.
Figures


References
-
- van der Tol IG, de Visscher JG, Jovanovic A, van der Waal I. Risk of second primary cancer following treatment of squamous cell carcinoma of the lower lip. Oral Oncol. 1999;35(6):571–574. - PubMed
-
- Koch WM, Stafford E, Bajaj G. Cancer of the oral cavity: General principles and management. In: Harrison LB, Sessions RB, Hong WK, editors. Head and Neck Cancer: A Multidisciplinary Approach. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009. pp. 250–264.
-
- Po Wing Yuen A, Lam KY, Lam LK, et al. Prognostic factors of clinically stage I and II oral tongue carcinoma-A comparative study of stage, thickness, shape, growth pattern, invasive front malignancy grading, Martinez-Gimeno score, and pathologic features. Head Neck. 2002;24(6):513–520. - PubMed
-
- Harrison LB, Sessions RB, Hong WK. Head and Neck Cancer: A Multidisciplinary Approach. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009.
-
- Smart JD. Recent developments in the use of bioadhesive systems for delivery of drugs to the oral cavity. Crit Rev Ther Drug Carrier Syst. 2004;21(4):319–344. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

