HDAC4 as a potential therapeutic target in neurodegenerative diseases: a summary of recent achievements
- PMID: 25759639
- PMCID: PMC4338808
- DOI: 10.3389/fncel.2015.00042
HDAC4 as a potential therapeutic target in neurodegenerative diseases: a summary of recent achievements
Abstract
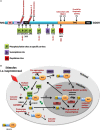
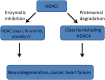
For the past decade protein acetylation has been shown to be a crucial post-transcriptional modification involved in the regulation of protein functions. Histone acetyltransferases (HATs) mediate acetylation of histones which results in the nucleosomal relaxation associated with gene expression. The reverse reaction, histone deacetylation, is mediated by histone deacetylases (HDACs) leading to chromatin condensation followed by transcriptional repression. HDACs are divided into distinct classes: I, IIa, IIb, III, and IV, on the basis of size and sequence homology, as well as formation of distinct repressor complexes. Implications of HDACs in many diseases, such as cancer, heart failure, and neurodegeneration, have identified these molecules as unique and attractive therapeutic targets. The emergence of HDAC4 among the members of class IIa family as a major player in synaptic plasticity raises important questions about its functions in the brain. The characterization of HDAC4 specific substrates and molecular partners in the brain will not only provide a better understanding of HDAC4 biological functions but also might help to develop new therapeutic strategies to target numerous malignancies. In this review we highlight and summarize recent achievements in understanding the biological role of HDAC4 in neurodegenerative processes.
Keywords: HDAC inhibitors; HDAC4; histone deacetylase; neurodegeneration; signaling; therapeutic potential.
Figures
References
-
- Akchiche N., Bossenmeyer-Pourie C., Kerek R., Martin N., Pourie G., Koziel V., et al. (2012). Homocysteinylation of neuronal proteins contributes to folate deficiency-associated alterations of differentiation, vesicular transport, and plasticity in hippocampal neuronal cells. FASEB J. 26, 3980–3992 10.1096/fj.12-205757 - DOI - PubMed
-
- Bongers K. S., Fox D. K., Ebert S. M., Kunkel S. D., Dyle M. C., Bullard S. A., et al. (2013). Skeletal muscle denervation causes skeletal muscle atrophy through a pathway that involves both Gadd45a and HDAC4. Am. J. Physiol. Endocrinol. Metab. 305, E907–E915. 10.1152/ajpendo.00380.2013 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

