Scalable and DiI-compatible optical clearance of the mammalian brain
- PMID: 25759641
- PMCID: PMC4338786
- DOI: 10.3389/fnana.2015.00019
Scalable and DiI-compatible optical clearance of the mammalian brain
Abstract
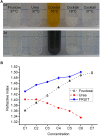
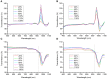
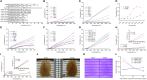
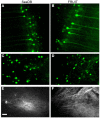
Efficient optical clearance is fundamental for whole brain imaging. In particular, clearance of the brain without membrane damage is required for the imaging of lipophilic tracer-labeled neural tracts. Relying on an ascending gradient of fructose solutions, SeeDB can achieve sufficient transparency of the mouse brain while ensuring that the plasma membrane remains intact. However, it is challenging to extend this method to larger mammalian brains due to the extremely high viscosity of the saturated fructose solution. Here we report a SeeDB-derived optical clearing method, termed FRUIT, which utilizes a cocktail of fructose and urea. As demonstrated in the adult mouse brain, combination of these two highly water-soluble clearing agents exerts a synergistic effect on clearance. More importantly, the final FRUIT solution has low viscosity so as to produce transparency of the whole adult rabbit brain via arterial perfusion, which is impossible to achieve with a saturated fructose solution. In addition to good compatibility with enhanced yellow fluorescent protein, the cocktail also preserves the fluorescence of the lipophilic tracer DiI. This work provides a volume-independent optical clearing method which retains the advantages of SeeDB, particularly compatibility with lipophilic tracers.
Keywords: 3DISCO; CLARITY; CUBIC; SeeDB; Urea; optical tissue clearing; tract tracing; whole brain imaging.
Figures


References
-
- Boutin M. E., Hoffman-Kim D. (2014). Application and assessment of optical clearing methods for imaging of tissue-engineered neural stem cell spheres. Tissue Eng. Part C Methods. [Epub ahead of print]. Available online at: http://online.liebertpub.com/doi/abs/10.1089/ten.TEC.2014.0296?url_ver=Z... 10.1089/ten.tec.2014.0296 - DOI - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

