A comparative evaluation of a novel vaccine in APP/PS1 mouse models of Alzheimer's disease
- PMID: 25759822
- PMCID: PMC4339718
- DOI: 10.1155/2015/807146
A comparative evaluation of a novel vaccine in APP/PS1 mouse models of Alzheimer's disease
Abstract
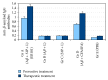
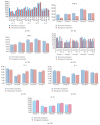
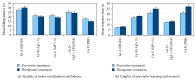
Immunization against amyloid-beta-peptide (Aβ) has been widely investigated as a potential immunotherapeutic approach for Alzheimer's disease (AD). With the aim of developing an active immunogenic vaccine without need of coadjuvant modification for human trials and therefore avoiding such side effects, we designed the Aβ 1-42 vaccine (EB101), delivered in a liposomal matrix, that based on our previous studies significantly prevents and reverses the AD neuropathology, clearing Aβ plaques while markedly reducing neuronal degeneration, behavioral deficits, and minimizing neuroinflammation in APP/PS1 transgenic mice. Here, the efficacy of our immunogenic vaccine EB101 was compared with the original immunization vaccine cocktail Aβ 42 + CFA/IFA (Freund's adjuvant), in order to characterize the effect of sphingosine-1-phosphate (S1P) in the immunotherapeutic response. Quantitative analysis of amyloid burden showed a notable decrease in the neuroinflammation reaction against Aβ plaques when S1P was compared with other treatments, suggesting that S1P plays a key role as a neuroprotective agent. Moreover, EB101 immunized mice presented a protective immunogenic reaction resulting in the increase of Aβ-specific antibody response and decrease of reactive glia in the affected brain areas, leading to a Th2 immunological reaction.
Figures





References
-
- Cacabelos R., Fernandez-Novoa L., Lombardi V., Kubota Y., Takeda M. Molecular genetics of Alzheimer's disease and aging. Methods and Findings in Experimental and Clinical Pharmacology. 2005;27:1–573. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

