Human CD38hiCD138⁺ plasma cells can be generated in vitro from CD40-activated switched-memory B lymphocytes
- PMID: 25759831
- PMCID: PMC4352507
- DOI: 10.1155/2014/635108
Human CD38hiCD138⁺ plasma cells can be generated in vitro from CD40-activated switched-memory B lymphocytes
Abstract
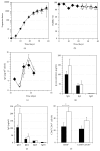
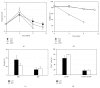
B lymphocyte differentiation into long-lived plasma cells is the keystone event for the production of long-term protective antibodies. CD40-CD154 and CD27-CD70 interactions are involved in human B lymphocyte differentiation into CD38(hi)CD138(+) cells in vivo as well as in vitro. In this study, we have compared these interactions in their capacity to drive switched-memory B lymphocytes differentiation into CD38(hi)CD138(+) plasma cells. The targeted B lymphocytes were isolated from human peripheral blood, expanded for 19 days, and then submitted to CD70 or CD154 interactions for 14 days. The expanded B lymphocytes were constitutively expressing CD39, whereas CD31's expression was noticed only following the in vitro differentiation step (day 5) and was exclusively present on the CD38(hi) cell population. Furthermore, the generated CD38(hi)CD138(+) cells showed a higher proportion of CD31(+) cells than the CD38(hi)CD138(-) cells. Besides, analyses done with human blood and bone marrow plasma cells showed that in vivo and de novo generated CD38(hi)CD138(+) cells have a similar CD31 expression profile but are distinct according to their reduced CD39 expression level. Overall, we have evidences that in vitro generated plasma cells are heterogeneous and appear as CD39(+) precursors to the ones present in bone marrow niches.
Figures




References
-
- Kawano M. M., Mihara K., Huang N., Tsujimoto T., Kuramoto A. Differentiation of early plasma cells on bone marrow stromal cells requires interleukin-6 for escaping from apoptosis. Blood. 1995;85(2):487–494. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

