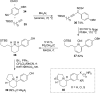
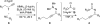Redox cycloisomerization approach to 1,2-dihydropyridines
- PMID: 25760318
- PMCID: PMC4883060
- DOI: 10.1021/acs.orglett.5b00279
Redox cycloisomerization approach to 1,2-dihydropyridines
Abstract
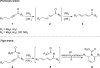
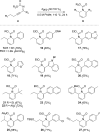
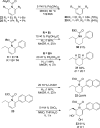
The phosphine-catalyzed synthesis of 1,2-dihydropyridines via an alkyne isomerization/electrocyclization sequence is described. Propargylidenecarbamate substrates were prepared following a one-pot procedure between a terminal alkyne, a benzonitrile, and a chloroformate in the presence of trimethylaluminum. This methodology gives access to a diverse set of 2,6-disubstituted 1,2-dihydropyridines in high yield. The products can be easily converted into substituted piperidines or pyridines, and this methodology was applied to the synthesis of indolizidines.
Figures
References
-
- Carey JS, Laffan D, Thomson C, Williams MT. Org. Biomol. Chem. 2006;4:2337. - PubMed
-
-
For a recent review on N-heterocycles synthesis, see: Vo TC-V, Bode JW. J. Org. Chem. 2014;79:2809. and references cited therein.
-
-
- Weintraub PM, Sabol JS, Kane JM, Borcherding DR. Tetrahedron. 2003;59:2953.
- Buffat MGP. Tetrahedron. 2004;60:1701.
- Kobayashi T, Sakaguchi T, Katsumura S. Heterocycles. 2013;87:729.
-
- Bull JA, Mousseau JJ, Pelletier G, Charette AB. Chem. Rev. 2012;112:2642. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources