Antibiotic treatment of severe exacerbations of chronic obstructive pulmonary disease with procalcitonin: a randomized noninferiority trial
- PMID: 25760346
- PMCID: PMC4356612
- DOI: 10.1371/journal.pone.0118241
Antibiotic treatment of severe exacerbations of chronic obstructive pulmonary disease with procalcitonin: a randomized noninferiority trial
Abstract
Background: The duration of antibiotic treatment of exacerbations of COPD (ECOPD) is controversial. Serum procalcitonin (PCT) is a biomarker of bacterial infection used to identify the cause of ECOPD.
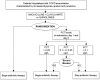
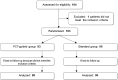
Methods and findings: We investigated whether a PCT-guided plan would allow a shorter duration of antibiotic treatment in patients with severe ECOPD. For this multicenter, randomized, non-inferiority trial, we enrolled 184 patients hospitalized with ECOPD from 18 hospitals in Italy. Patients were assigned to receive antibiotics for 10 days (standard group) or for either 3 or 10 days (PCT group). The primary outcome was the rate of ECOPD at 6 months. Having planned to recruit 400 patients, we randomized only 183: 93 in the PCT group and 90 in the standard group. Thus, the completed study was underpowered. The ECOPD rate at 6 months between PCT-guided and standard antibiotic treatment was not significant (% difference, 4.04; 90% confidence interval [CI], -7.23 to 15.31), but the CI included the non-inferiority margin of 15. In the PCT-guided group, about 50% of patients were treated for 3 days, and there was no difference in primary or secondary outcomes compared to patients treated for 10 days.
Conclusions: Although the primary and secondary clinical outcomes were no different for patients treated for 3 or 10 days in the PCT group, the conclusion that antibiotics can be safely stopped after 3 days in patients with low serum PCT cannot be substantiated statistically. Thus, the results of this study are inconclusive regarding the noninferiority of the PCT-guided plan compared to the standard antibiotic treatment. The study was funded by Agenzia Italiana del Farmaco (AIFA-FARM58J2XH). Clinical trial registered with www.clinicaltrials.gov (NCT01125098).
Trial registration: ClinicalTrials.gov NCT01125098.
Conflict of interest statement
Figures

Similar articles
-
Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial.JAMA. 2009 Sep 9;302(10):1059-66. doi: 10.1001/jama.2009.1297. JAMA. 2009. PMID: 19738090 Clinical Trial.
-
Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy.Chest. 2007 Jan;131(1):9-19. doi: 10.1378/chest.06-1500. Chest. 2007. PMID: 17218551 Clinical Trial.
-
Procalcitonin guided antibiotic therapy and hospitalization in patients with lower respiratory tract infections: a prospective, multicenter, randomized controlled trial.BMC Health Serv Res. 2007 Jul 5;7:102. doi: 10.1186/1472-6963-7-102. BMC Health Serv Res. 2007. PMID: 17615073 Free PMC article. Clinical Trial.
-
Procalcitonin-guided antibiotic therapy for chronic obstructive pulmonary disease exacerbations.Expert Rev Anti Infect Ther. 2011 Jun;9(6):727-35. doi: 10.1586/eri.11.45. Expert Rev Anti Infect Ther. 2011. PMID: 21692680 Review.
-
Procalcitonin to guide antibiotic therapy in the ICU.Int J Antimicrob Agents. 2015 Dec;46 Suppl 1:S19-24. doi: 10.1016/j.ijantimicag.2015.10.012. Epub 2015 Nov 1. Int J Antimicrob Agents. 2015. PMID: 26607343 Review.
Cited by
-
The role of acute and chronic respiratory colonization and infections in the pathogenesis of COPD.Respirology. 2017 May;22(4):634-650. doi: 10.1111/resp.13032. Epub 2017 Mar 25. Respirology. 2017. PMID: 28342288 Free PMC article. Review.
-
Procalcitonin to guide antibiotic administration in COPD exacerbations: a meta-analysis.Eur Respir Rev. 2017 Jan 31;26(143):160073. doi: 10.1183/16000617.0073-2016. Print 2017 Jan. Eur Respir Rev. 2017. PMID: 28143877 Free PMC article. Review.
-
Predictive Value of Procalcitonin for Infection and Survival in Adult Cardiogenic Shock Patients Treated with Extracorporeal Membrane Oxygenation.Chonnam Med J. 2018 Jan;54(1):48-54. doi: 10.4068/cmj.2018.54.1.48. Epub 2018 Jan 25. Chonnam Med J. 2018. PMID: 29399566 Free PMC article.
-
Adapting Drug Approval Pathways for Bacteriophage-Based Therapeutics.Front Microbiol. 2016 Aug 3;7:1209. doi: 10.3389/fmicb.2016.01209. eCollection 2016. Front Microbiol. 2016. PMID: 27536293 Free PMC article. Review.
-
2021 Guideline for the Management of COPD Exacerbations: Emergency Medicine Association of Turkey (EMAT) / Turkish Thoracic Society (TTS) Clinical Practice Guideline Task Force.Turk J Emerg Med. 2021 Oct 29;21(4):137-176. doi: 10.4103/2452-2473.329630. eCollection 2021 Oct-Dec. Turk J Emerg Med. 2021. PMID: 34849428 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

