Compliance with results reporting at ClinicalTrials.gov
- PMID: 25760355
- PMCID: PMC4508873
- DOI: 10.1056/NEJMsa1409364
Compliance with results reporting at ClinicalTrials.gov
Abstract
Background: The Food and Drug Administration Amendments Act (FDAAA) mandates timely reporting of results of applicable clinical trials to ClinicalTrials.gov. We characterized the proportion of applicable clinical trials with publicly available results and determined independent factors associated with the reporting of results.
Methods: Using an algorithm based on input from the National Library of Medicine, we identified trials that were likely to be subject to FDAAA provisions (highly likely applicable clinical trials, or HLACTs) from 2008 through 2013. We determined the proportion of HLACTs that reported results within the 12-month interval mandated by the FDAAA or at any time during the 5-year study period. We used regression models to examine characteristics associated with reporting at 12 months and throughout the 5-year study period.
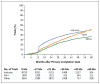
Results: From all the trials at ClinicalTrials.gov, we identified 13,327 HLACTs that were terminated or completed from January 1, 2008, through August 31, 2012. Of these trials, 77.4% were classified as drug trials. A total of 36.9% of the trials were phase 2 studies, and 23.4% were phase 3 studies; 65.6% were funded by industry. Only 13.4% of trials reported summary results within 12 months after trial completion, whereas 38.3% reported results at any time up to September 27, 2013. Timely reporting was independently associated with factors such as FDA oversight, a later trial phase, and industry funding. A sample review suggested that 45% of industry-funded trials were not required to report results, as compared with 6% of trials funded by the National Institutes of Health (NIH) and 9% of trials that were funded by other government or academic institutions.
Conclusions: Despite ethical and legal obligations to disclose findings promptly, most HLACTs did not report results to ClinicalTrials.gov in a timely fashion during the study period. Industry-funded trials adhered to legal obligations more often than did trials funded by the NIH or other government or academic institutions. (Funded by the Clinical Trials Transformation Initiative and the NIH.).
Figures
Comment in
-
Most clinical trials fail to meet FDA requirement to publish results within a year.BMJ. 2015 Mar 12;350:h1333. doi: 10.1136/bmj.h1333. BMJ. 2015. PMID: 25767061 No abstract available.
-
Clinical trials: A transparent future for clinical trial reporting.Nat Rev Rheumatol. 2015 Jun;11(6):324-6. doi: 10.1038/nrrheum.2015.65. Epub 2015 May 5. Nat Rev Rheumatol. 2015. PMID: 25939418 No abstract available.
-
Compliance with results reporting at ClinicalTrials.gov.N Engl J Med. 2015 Jun 11;372(24):2370-1. doi: 10.1056/NEJMc1504513. N Engl J Med. 2015. PMID: 26061853 No abstract available.
-
Compliance with results reporting at ClinicalTrials.gov.N Engl J Med. 2015 Jun 11;372(24):2370. doi: 10.1056/NEJMc1504513. N Engl J Med. 2015. PMID: 26061854 No abstract available.
References
-
- Kesselheim AS, Mello MM. Confidentiality laws and secrecy in medical research: improving public access to data on drug safety. Health Aff (Millwood) 2007;26:483–91. - PubMed
-
- Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008;358:252–60. - PubMed
-
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–71. - PubMed
-
-
Food and Drug Administration Amendments Act of 2007. (Public Law No. 110–85 § 801;2007.)
-
-
- ClinicalTrials.gov. FDAAA 801 requirements. ( http://clinicaltrials.gov/ct2/manage-recs/fdaaa)
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
