Snapshots of ligand entry, malleable binding and induced helical movement in P-glycoprotein
- PMID: 25760620
- PMCID: PMC4356375
- DOI: 10.1107/S1399004715000978
Snapshots of ligand entry, malleable binding and induced helical movement in P-glycoprotein
Abstract
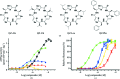
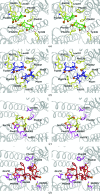
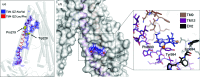
P-glycoprotein (P-gp) is a transporter of great clinical and pharmacological significance. Several structural studies of P-gp and its homologs have provided insights into its transport cycle, but questions remain regarding how P-gp recognizes diverse substrates and how substrate binding is coupled to ATP hydrolysis. Here, four new P-gp co-crystal structures with a series of rationally designed ligands are presented. It is observed that the binding of certain ligands, including an ATP-hydrolysis stimulator, produces a large conformational change in the fourth transmembrane helix, which is positioned to potentially transmit a signal to the nucleotide-binding domains. A new ligand-binding site on the surface of P-gp facing the inner leaflet of the membrane is also described, providing vital insights regarding the entry mechanism of hydrophobic drugs and lipids into P-gp. These results represent significant advances in the understanding of how P-gp and related transporters bind and export a plethora of metabolites, antibiotics and clinically approved and pipeline drugs.
Keywords: P-glycoprotein.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

