Diaphragm muscle fiber weakness and ubiquitin-proteasome activation in critically ill patients
- PMID: 25760684
- PMCID: PMC4451621
- DOI: 10.1164/rccm.201412-2214OC
Diaphragm muscle fiber weakness and ubiquitin-proteasome activation in critically ill patients
Abstract
Rationale: The clinical significance of diaphragm weakness in critically ill patients is evident: it prolongs ventilator dependency, and increases morbidity and duration of hospital stay. To date, the nature of diaphragm weakness and its underlying pathophysiologic mechanisms are poorly understood.
Objectives: We hypothesized that diaphragm muscle fibers of mechanically ventilated critically ill patients display atrophy and contractile weakness, and that the ubiquitin-proteasome pathway is activated in the diaphragm.
Methods: We obtained diaphragm muscle biopsies from 22 critically ill patients who received mechanical ventilation before surgery and compared these with biopsies obtained from patients during thoracic surgery for resection of a suspected early lung malignancy (control subjects). In a proof-of-concept study in a muscle-specific ring finger protein-1 (MuRF-1) knockout mouse model, we evaluated the role of the ubiquitin-proteasome pathway in the development of contractile weakness during mechanical ventilation.
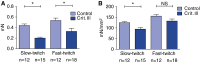
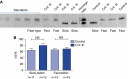
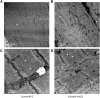
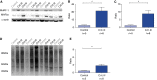
Measurements and main results: Both slow- and fast-twitch diaphragm muscle fibers of critically ill patients had approximately 25% smaller cross-sectional area, and had contractile force reduced by half or more. Markers of the ubiquitin-proteasome pathway were significantly up-regulated in the diaphragm of critically ill patients. Finally, MuRF-1 knockout mice were protected against the development of diaphragm contractile weakness during mechanical ventilation.
Conclusions: These findings show that diaphragm muscle fibers of critically ill patients display atrophy and severe contractile weakness, and in the diaphragm of critically ill patients the ubiquitin-proteasome pathway is activated. This study provides rationale for the development of treatment strategies that target the contractility of diaphragm fibers to facilitate weaning.
Keywords: diaphragm weakness; mechanical ventilation; single muscle fiber; weaning failure.
Figures





Comment in
-
Muscle weakness in critical illness.Am J Respir Crit Care Med. 2015 May 15;191(10):1094-6. doi: 10.1164/rccm.201503-0478ED. Am J Respir Crit Care Med. 2015. PMID: 25978566 No abstract available.
References
-
- Batt J, Dos Santos CC, Herridge MS. Muscle injury during critical illness. JAMA. 2013;310:1569–1570. - PubMed
-
- Schweickert WD, Hall J. ICU-acquired weakness. Chest. 2007;131:1541–1549. - PubMed
-
- Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE, et al. Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. - PubMed
-
- De Jonghe B, Bastuji-Garin S, Sharshar T, Outin H, Brochard L. Does ICU-acquired paresis lengthen weaning from mechanical ventilation? Intensive Care Med. 2004;30:1117–1121. - PubMed
-
- Boles J-M, Bion J, Connors A, Herridge M, Marsh B, Melot C, Pearl R, Silverman H, Stanchina M, Vieillard-Baron A, et al. Weaning from mechanical ventilation. Eur Respir J. 2007;29:1033–1056. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

