Examining the feasibility of clinical grade CD271+ enrichment of mesenchymal stromal cells for bone regeneration
- PMID: 25760857
- PMCID: PMC4356586
- DOI: 10.1371/journal.pone.0117855
Examining the feasibility of clinical grade CD271+ enrichment of mesenchymal stromal cells for bone regeneration
Abstract
Introduction: Current clinical trials utilize mesenchymal stromal cells (MSCs) expanded in culture, however these interventions carry considerable costs and concerns pertaining to culture-induced losses of potency. This study assessed the feasibility of new clinical-grade technology to obtain uncultured MSC isolates from three human intra-osseous tissue sources based on immunomagnetic selection for CD271-positive cells.
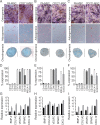
Materials and methods: MSCs were isolated from bone marrow (BM) aspirates or surgical waste materials; enzymatically digested femoral heads (FHs) and reamer irrigator aspirator (RIA) waste fluids. Flow cytometry for the CD45-/lowCD73+CD271+ phenotype was used to evaluate uncultured MSCs before and after selection, and to measure MSC enrichment in parallel to colony forming-unit fibroblast assay. Trilineage differentiation assays and quantitative polymerase chain-reaction for key transcripts involved in bone regeneration was used to assess the functional utility of isolated cells for bone repair.
Results: Uncultured CD45-/lowCD271+ MSCs uniformly expressed CD73, CD90 and CD105 but showed variable expression of MSCA-1 and SUSD2 (BM>RIA>FH). MSCs were enriched over 150-fold from BM aspirates and RIA fluids, whereas the highest MSC purities were obtained from FH digests. Enriched fractions expressed increased levels of BMP-2, COL1A2, VEGFC, SPARC and CXCL12 transcripts (BM>RIA>FH), with the highest up-regulation detected for CXCL12 in BM (>1300-fold). Following culture expansion, CD271-selected MSCS were tri-potential and phenotypically identical to plastic adherence-selected MSCs.
Discussion: A CD271-based GMP-compliant immunomagnetic selection resulted in a substantial increase in MSC purity and elevated expression of transcripts involved in bone formation, vascularisation and chemo-attraction. Although this technology, particularly from RIA fluids, can be immediately applied by orthopaedic surgeons as autologous therapy, further improvements in MSC purities and pre-clinical testing of product safety would be required to develop this process for allogeneic applications.
Conflict of interest statement
Figures



References
-
- Haleem AM, Singergy AA, Sabry D, Atta HM, Rashed LA, et al. (2010) The Clinical Use of Human Culture-Expanded Autologous Bone Marrow Mesenchymal Stem Cells Transplanted on Platelet-Rich Fibrin Glue in the Treatment of Articular Cartilage Defects: A Pilot Study and Preliminary Results. Cartilage 1: 253–261. - PMC - PubMed
-
- Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, et al. (2009) A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol 54: 2277–2286. 10.1016/j.jacc.2009.06.055 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

