Enhanced neuronal glucose transporter expression reveals metabolic choice in a HD Drosophila model
- PMID: 25761110
- PMCID: PMC4356621
- DOI: 10.1371/journal.pone.0118765
Enhanced neuronal glucose transporter expression reveals metabolic choice in a HD Drosophila model
Abstract
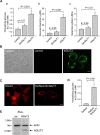
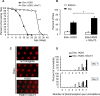
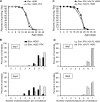
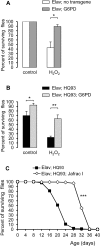
Huntington's disease is a neurodegenerative disorder caused by toxic insertions of polyglutamine residues in the Huntingtin protein and characterized by progressive deterioration of cognitive and motor functions. Altered brain glucose metabolism has long been suggested and a possible link has been proposed in HD. However, the precise function of glucose transporters was not yet determined. Here, we report the effects of the specifically-neuronal human glucose transporter expression in neurons of a Drosophila model carrying the exon 1 of the human huntingtin gene with 93 glutamine repeats (HQ93). We demonstrated that overexpression of the human glucose transporter in neurons ameliorated significantly the status of HD flies by increasing their lifespan, reducing their locomotor deficits and rescuing eye neurodegeneration. Then, we investigated whether increasing the major pathways of glucose catabolism, glycolysis and pentose-phosphate pathway (PPP) impacts HD. To mimic increased glycolytic flux, we overexpressed phosphofructokinase (PFK) which catalyzes an irreversible step in glycolysis. Overexpression of PFK did not affect HQ93 fly survival, but protected from photoreceptor loss. Overexpression of glucose-6-phosphate dehydrogenase (G6PD), the key enzyme of the PPP, extended significantly the lifespan of HD flies and rescued eye neurodegeneration. Since G6PD is able to synthesize NADPH involved in cell survival by maintenance of the redox state, we showed that tolerance to experimental oxidative stress was enhanced in flies co-expressing HQ93 and G6PD. Additionally overexpressions of hGluT3, G6PD or PFK were able to circumvent mitochondrial deficits induced by specific silencing of genes necessary for mitochondrial homeostasis. Our study confirms the involvement of bioenergetic deficits in HD course; they can be rescued by specific expression of a glucose transporter in neurons. Finally, the PPP and, to a lesser extent, the glycolysis seem to mediate the hGluT3 protective effects, whereas, in addition, the PPP provides increased protection to oxidative stress.
Conflict of interest statement
Figures


References
-
- Jeong SJ, Kim M, Chang KA, Kim HS, Park CH, Suh YH. Huntingtin is localized in the nucleus during preimplanatation embryo development in mice. Int J Dev Neurosci. 2006; 24: 81–85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

