Inhibition of ABCA1 protein degradation promotes HDL cholesterol efflux capacity and RCT and reduces atherosclerosis in mice
- PMID: 25761370
- PMCID: PMC4409288
- DOI: 10.1194/jlr.M054742
Inhibition of ABCA1 protein degradation promotes HDL cholesterol efflux capacity and RCT and reduces atherosclerosis in mice
Abstract
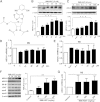
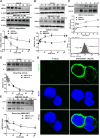
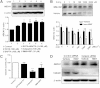
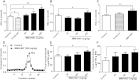
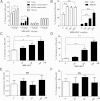
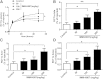
ABCA1 plays a key role in the initial lipidation of apoA-I, which generates circulating HDL cholesterol. Whereas it is known that the transcriptional upregulation of ABCA1 promotes HDL formation and reverse cholesterol transport (RCT), it is not known how the inhibition of ABCA1 protein degradation impacts HDL function. Employing the small molecule triacetyl-3-hydroxyphenyladenosine (IMM-H007), we determined how the attenuation of ABCA1 protein degradation affects HDL cholesterol efflux capacity, RCT, and atherosclerotic lesion formation. Pulse-chase analysis revealed that IMM-H007 inhibits ABCA1 degradation and facilitates its cell-surface localization in macrophages, and additional studies in macrophages showed that IMM-H007 thereby promotes cholesterol efflux. IMM-H007 treatment of Paigen diet-fed mice caused an increase in circulating HDL level, it increased the cholesterol efflux capacity of HDL, and it enhanced in vivo RCT from macrophages to the plasma, liver, and feces. Furthermore, ABCA1 degradation suppression by IMM-H007 reduced atherosclerotic plaque formation in apoE(-/-) mice. Thus, via effects on both ABCA1-expressing cells and circulating HDL function, the inhibition of ABCA1 protein degradation by IMM-H007 promotes HDL cholesterol efflux capacity and RCT and attenuates atherogenesis. IMM-H007 potentially represents a lead compound for the development of agents to augment HDL function.
Keywords: ATP binding cassette transporter A1; high density lipoprotein; macrophage; reverse cholesterol transport.
Copyright © 2015 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
-
- Wilson P. W. 1990. High-density lipoprotein, low-density lipoprotein and coronary artery disease. Am. J. Cardiol. 66: 7A–10A. - PubMed
-
- Rader D. J., Tall A. R. 2012. The not-so-simple HDL story: is it time to revise the HDL cholesterol hypothesis? Nat. Med. 18: 1344–1346. - PubMed
-
- Rubin E. M., Krauss R. M., Spangler E. A., Verstuyft J. G., Clift S. M. 1991. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature. 353: 265–267. - PubMed
-
- Boden W. E., Probstfield J. L., Anderson T., Chaitman B. R., Desvignes-Nickens P., Koprowicz K., McBride R., Teo K., Weintraub W.; AIM-HIGH Investigators. 2011. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 365: 2255–2267. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

