Efficacy and tolerability of 16% subcutaneous immunoglobulin compared with 20% subcutaneous immunoglobulin in primary antibody deficiency
- PMID: 25761372
- PMCID: PMC4557380
- DOI: 10.1111/cei.12623
Efficacy and tolerability of 16% subcutaneous immunoglobulin compared with 20% subcutaneous immunoglobulin in primary antibody deficiency
Abstract
Multiple subcutaneous immunoglobulin (SCIG) products are available to treat primary antibody deficiency (PAD). The efficacy and tolerability of 16% SCIG (Vivaglobin(®) ) was compared with 20% SCIG (Hizentra(®) ) in PAD subjects. The study was a prospective, single-centre, open-label study of PAD subjects transitioning Vivaglobin to equivalent Hizentra doses, rounded to the nearest vial size. Comparisons included immunoglobulin (Ig)G levels; tetanus, varicella and Streptococcus pneumoniae titres; adverse events (AEs), annual infection rate and quality of life during 8 weeks of Vivaglobin and 24 weeks of Hizentra. Thirty-two subjects (aged 2-75 years) participated. Rounding to the nearest Hizentra vial size resulted in a 12·8% (± 2·9%) increase in SCIG dose. Median immunoglobulin (Ig)G level following 8 weeks of Vivaglobin was similar to 24 weeks of Hizentra (1050 versus 1035 mg/dl, respectively; P = 0·77). Both products had similar protective titres to tetanus, varicella and serotypes of S. pneumoniae, which were variable but well above protective levels. After 12 weeks of Hizentra, subjects reported fewer local site reactions compared with Vivaglobin. Switching products resulted in increased systemic AEs in some subjects but, overall, not significantly higher than during Vivaglobin treatment. Average infusion time decreased from 104·7 min (3·3 sites) with Vivaglobin to 70·7 min (2·2 sites) with Hizentra (P = 0·0005). Acute serious bacterial infections were similar. Treatment satisfaction was superior with Hizentra. Hizentra and Vivaglobin have similar pharmacokinetics and efficacy. Although transition to a different SCIG product initially increased AEs, Hizentra is well tolerated and can be infused more rapidly and with fewer sites compared to Vivaglobin.
Keywords: Hizentra; IVIG; IgG; Vivaglobin; antibody deficiency; immunodeficiency; pneumococcal titres; quality of life; subcutaneous immunoglobulin; varicella.
© 2015 British Society for Immunology.
Figures
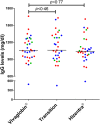
 ), common variable immunodeficiency or X-linked IgM syndrome (XLHIGM) (
), common variable immunodeficiency or X-linked IgM syndrome (XLHIGM) ( ) and specific antibody deficiency or IgG subclass deficiency (
) and specific antibody deficiency or IgG subclass deficiency ( ) are shown. Median steady-state IgG levels are shown by solid lines, with P-value comparisons between Vivaglobin and Hizentra at week 20 and Vivaglobin and Hizentra at week 32 using a paired t-test.
) are shown. Median steady-state IgG levels are shown by solid lines, with P-value comparisons between Vivaglobin and Hizentra at week 20 and Vivaglobin and Hizentra at week 32 using a paired t-test.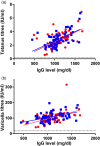
 ) treatment and week 32 during Hizentra (
) treatment and week 32 during Hizentra ( ) treatment. Cut-off values for protective titre levels are shown by dashed lines. Significant Spearman's correlations were present for both subcutaneous immunoglobulin (SCIG) products between IgG steady-state level and titres for tetanus (Vivaglobin, R = 0·59, P < 0·0001; Hizentra, R = 0·58, P < 0·0001) and varicella (Vivaglobin, R = 0·57, P < 0·0001; Hizentra, R = 0·45, P = 0·0002).
) treatment. Cut-off values for protective titre levels are shown by dashed lines. Significant Spearman's correlations were present for both subcutaneous immunoglobulin (SCIG) products between IgG steady-state level and titres for tetanus (Vivaglobin, R = 0·59, P < 0·0001; Hizentra, R = 0·58, P < 0·0001) and varicella (Vivaglobin, R = 0·57, P < 0·0001; Hizentra, R = 0·45, P = 0·0002).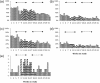
 ), the first 12 weeks of Hizentra (
), the first 12 weeks of Hizentra ( ) and the final 12 weeks of Hizentra (
) and the final 12 weeks of Hizentra ( ) treatment. Average weekly percentages of subjects with local site reactions of oedema (a), erythema (b), pain (c) or pruritus (d) are shown. (e) The number of individual systemic adverse events (AEs) reported for each week of study on the y-axis, defined as fever, myalgia, headache, or malaise within 72 h of the infusion. Solid arrows designate P < 0·05, while dashed arrows designate no significant differences (Mann–Whitney rank sum test).
) treatment. Average weekly percentages of subjects with local site reactions of oedema (a), erythema (b), pain (c) or pruritus (d) are shown. (e) The number of individual systemic adverse events (AEs) reported for each week of study on the y-axis, defined as fever, myalgia, headache, or malaise within 72 h of the infusion. Solid arrows designate P < 0·05, while dashed arrows designate no significant differences (Mann–Whitney rank sum test).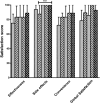
 ) treatment, week 8 on Vivaglobin (
) treatment, week 8 on Vivaglobin ( ) treatment, week 12 on Hizentra (
) treatment, week 12 on Hizentra ( ) treatment, week 24 on Hizentra (
) treatment, week 24 on Hizentra ( ) treatment and week 32 on Hizentra (
) treatment and week 32 on Hizentra ( ) treatment. A significant improvement in the satisfaction scores of side effects was found between week 8 on Vivaglobin treatment and week 32 on Hizentra treatment (P = 0·017;Wilcoxon matched-pairs signed-rank test).
) treatment. A significant improvement in the satisfaction scores of side effects was found between week 8 on Vivaglobin treatment and week 32 on Hizentra treatment (P = 0·017;Wilcoxon matched-pairs signed-rank test).References
-
- Bonilla FA, Bernstein IL, Khan DA, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. 2005;94:S1–63. Ann Allergy Asthma Immunol. - PubMed
-
- Orange JS, Hossny EM, Weiler CR, et al. Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. 2006;117:S525–53. J Allergy Clin Immunol. - PubMed
-
- Jolles S, Bernatowska E, de Gracia J, et al. Efficacy and safety of Hizentra((R)) in patients with primary immunodeficiency after a dose-equivalent switch from intravenous or subcutaneous replacement therapy. Clin Immunol. 2011;141:90–102. - PubMed
-
- Berger M. Subcutaneous immunoglobulin replacement in primary immunodeficiencies. Clin Immunol. 2004;112:1–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

