The Efficacy and Safety of Platinum/Vinorelbine as More Than Second-Line Chemotherapy for Advanced Non-small Cell Lung Cancer
- PMID: 25761490
- PMCID: PMC4614189
- DOI: 10.4143/crt.2014.316
The Efficacy and Safety of Platinum/Vinorelbine as More Than Second-Line Chemotherapy for Advanced Non-small Cell Lung Cancer
Abstract
Purpose: There is no regimen that is strongly recommended for more than second-line treatment. We investigated the efficacy and safety of platinum/vinorelbine as more than second-line treatment.
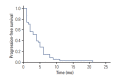
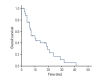
Materials and methods: We selected patients with advanced non-small cell lung cancer (NSCLC) who received treatment with platinum/vinorelbine at Chungnam National University Hospital from August 2001 to December 2013. The primary end point was the response rate, and secondary end points were progression-free survival (PFS), overall survival (OS), and toxicity.
Results: Thirty-five patients were enrolled. Response rate was 22.9% (complete response, 0 patients [0%]; partial response, eight patients [22.9%]; stable disease, 10 patients [28.6%]; progressive disease, 14 patients [40.0%]). A significantly higher response rate was observed for patients who had responded to previous chemotherapy than for those who did not (34.8% [8/23] vs. 0% [0/12], p=0.020). The median PFS was 4 months (range, 1 to 21 months). Patients with adenocarcinoma and non-smokers had a significantly longer PFS than patients with non-adenocarcinoma and smokers (5 months vs. 2 months, p=0.007; 4.5 months vs. 2 months, p=0.046, respectively). The median OS was 10 months (range, 1 to 41 months). Patients with good performance status and non-smokers had a significantly longer OS than patients with poor performance status and smokers (14 months vs. 4 months, p=0.02; 18.5 months vs. 6 months, p=0.049, respectively). The main serious adverse event (grade 3 or 4) was neutropenia (15 events, 13.3%) in a total of 113 cycles.
Conclusion: Platinum/vinorelbine was effective as more than second-line chemotherapy, and the toxicity was tolerable, in patients with advanced NSCLC.
Keywords: Drug therapy; Non-small-cell lung carcinoma; Third-line treatment.
Conflict of interest statement
Conflict of interest relevant to this article was not reported.
Figures
References
-
- Asahina H, Sekine I, Horinouchi H, Nokihara H, Yamamoto N, Kubota K, et al. Retrospective analysis of third-line and fourth-line chemotherapy for advanced non-small-cell lung cancer. Clin Lung Cancer. 2012;13:39–43. - PubMed
-
- Girard N, Jacoulet P, Gainet M, Elleuch R, Pernet D, Depierre A, et al. Third-line chemotherapy in advanced non-small cell lung cancer: identifying the candidates for routine practice. J Thorac Oncol. 2009;4:1544–9. - PubMed
-
- National Comprehensive Cancer Network . Fort Washington, PA: National Comprehensive Cancer Network; c2015. NCCN clinical practice guidelines in oncology. Non-small cell lung cancer version 3.2014 [Internet] [cited 2014 Nov 3]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. - PubMed
-
- Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O'Rourke M, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–103. - PubMed
-
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical