TLR-independent anti-inflammatory function of intestinal epithelial TRAF6 signalling prevents DSS-induced colitis in mice
- PMID: 25761602
- PMCID: PMC4893119
- DOI: 10.1136/gutjnl-2014-308323
TLR-independent anti-inflammatory function of intestinal epithelial TRAF6 signalling prevents DSS-induced colitis in mice
Abstract
Objective: The gut microbiota modulates host susceptibility to intestinal inflammation, but the cell types and the signalling pathways orchestrating this bacterial regulation of intestinal homeostasis remain poorly understood. Here, we investigated the function of intestinal epithelial toll-like receptor (TLR) responses in the dextran sodium sulfate (DSS)-induced mouse model of colitis.
Design: We applied an in vivo genetic approach allowing intestinal epithelial cell (IEC)-specific deletion of the critical TLR signalling adaptors, MyD88 and/or TIR-domain-containing adapter-inducing interferon-β (TRIF), as well as the downstream ubiquitin ligase TRAF6 in order to reveal the IEC-intrinsic function of these TLR signalling molecules during DSS colitis.
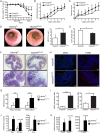
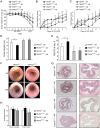
Results: Mice lacking TRAF6 in IECs showed exacerbated DSS-induced inflammatory responses that ensued in the development of chronic colon inflammation. Antibiotic pretreatment abolished the increased DSS susceptibility of these mice, showing that epithelial TRAF6 signalling pathways prevent the gut microbiota from driving excessive colitis. However, in contrast to epithelial TRAF6 deletion, blocking epithelial TLR signalling by simultaneous deletion of MyD88 and TRIF specifically in IECs did not affect DSS-induced colitis severity. This in vivo functional comparison between TRAF6 and MyD88/TRIF deletion in IECs shows that the colitis-protecting effects of epithelial TRAF6 signalling are not triggered by TLRs.
Conclusions: Intestinal epithelial TRAF6-dependent but MyD88/TRIF-independent and, thus, TLR-independent signalling pathways are critical for preventing propagation of DSS-induced colon inflammation by the gut microbiota. Moreover, our experiments using mice with dual MyD88/TRIF deletion in IECs unequivocally show that the gut microbiota trigger non-epithelial TLRs rather than epithelial TLRs to restrict DSS colitis severity.
Keywords: EXPERIMENTAL COLITIS; GUT INFLAMMATION.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
