International Union of Basic and Clinical Pharmacology. XCV. Recent advances in the understanding of the pharmacology and biological roles of relaxin family peptide receptors 1-4, the receptors for relaxin family peptides
- PMID: 25761609
- PMCID: PMC4394689
- DOI: 10.1124/pr.114.009472
International Union of Basic and Clinical Pharmacology. XCV. Recent advances in the understanding of the pharmacology and biological roles of relaxin family peptide receptors 1-4, the receptors for relaxin family peptides
Abstract
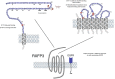
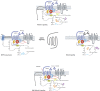
Relaxin, insulin-like peptide 3 (INSL3), relaxin-3, and INSL5 are the cognate ligands for the relaxin family peptide (RXFP) receptors 1-4, respectively. RXFP1 activates pleiotropic signaling pathways including the signalosome protein complex that facilitates high-sensitivity signaling; coupling to Gα(s), Gα(i), and Gα(o) proteins; interaction with glucocorticoid receptors; and the formation of hetero-oligomers with distinctive pharmacological properties. In addition to relaxin-related ligands, RXFP1 is activated by Clq-tumor necrosis factor-related protein 8 and by small-molecular-weight agonists, such as ML290 [2-isopropoxy-N-(2-(3-(trifluoromethylsulfonyl)phenylcarbamoyl)phenyl)benzamide], that act allosterically. RXFP2 activates only the Gα(s)- and Gα(o)-coupled pathways. Relaxin-3 is primarily a neuropeptide, and its cognate receptor RXFP3 is a target for the treatment of depression, anxiety, and autism. A variety of peptide agonists, antagonists, biased agonists, and an allosteric modulator target RXFP3. Both RXFP3 and the related RXFP4 couple to Gα(i)/Gα(o) proteins. INSL5 has the properties of an incretin; it is secreted from the gut and is orexigenic. The expression of RXFP4 in gut, adipose tissue, and β-islets together with compromised glucose tolerance in INSL5 or RXFP4 knockout mice suggests a metabolic role. This review focuses on the many advances in our understanding of RXFP receptors in the last 5 years, their signal transduction mechanisms, the development of novel compounds that target RXFP1-4, the challenges facing the field, and current prospects for new therapeutics.
Copyright © 2015 by The American Society for Pharmacology and Experimental Therapeutics.
Figures



References
-
- Adham IM, Burkhardt E, Benahmed M, Engel W. (1993) Cloning of a cDNA for a novel insulin-like peptide of the testicular Leydig cells. J Biol Chem 268:26668–26672. - PubMed
-
- Ahokas RA, Sibai BM, Anderson GD. (1989) Lack of evidence of a vasodepressor role for relaxin in spontaneously hypertensive and normotensive pregnant rats. Am J Obstet Gynecol 161:618–622. - PubMed
-
- Ahrén B. (2009) Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov 8:369–385. - PubMed
-
- Alexander BT, Miller MT, Kassab S, Novak J, Reckelhoff JF, Kruckeberg WC, Granger JP. (1999) Differential expression of renal nitric oxide synthase isoforms during pregnancy in rats. Hypertension 33:435–439. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

