TRPM7 inhibitor carvacrol protects brain from neonatal hypoxic-ischemic injury
- PMID: 25761704
- PMCID: PMC4337201
- DOI: 10.1186/s13041-015-0102-5
TRPM7 inhibitor carvacrol protects brain from neonatal hypoxic-ischemic injury
Abstract
Background: Our previous study found that suppression of TRPM7 reduced neuronal death in adult rat ischemic brain injury. It was reported that carvacrol blocked TRPM7 and attenuated brain injury in an adult rat MCAO model. The effects of carvacrol on neonatal stroke remain unknown. This study investigated the effects of carvacrol on neuronal injury and behavioral impairment after hypoxia-ischemia in neonatal mice and the potential signaling pathway underlying these effects.
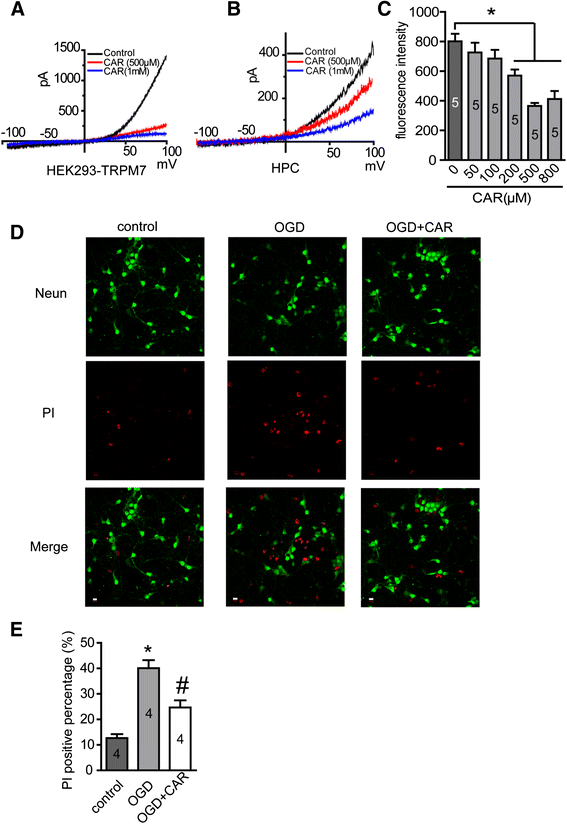
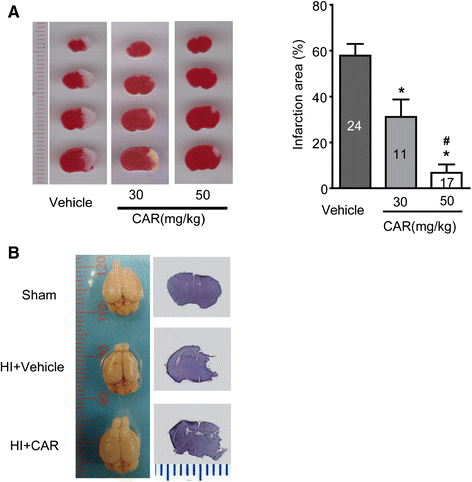
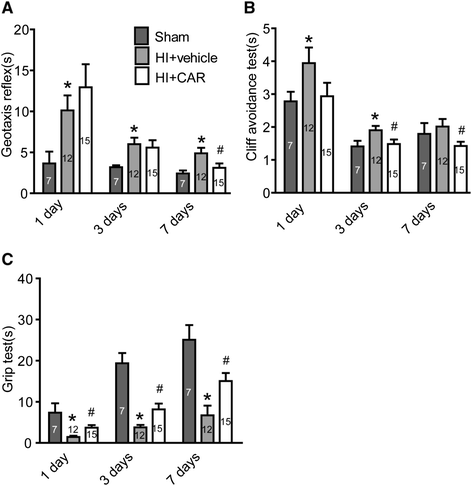
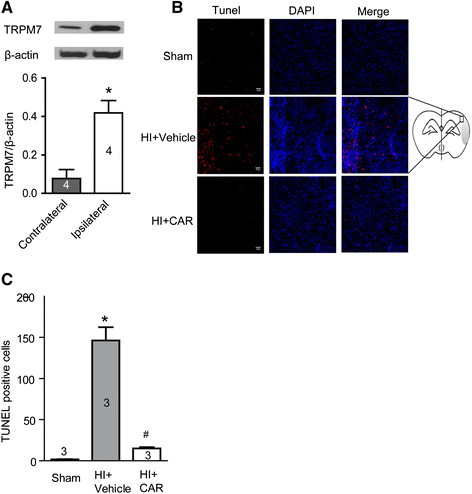
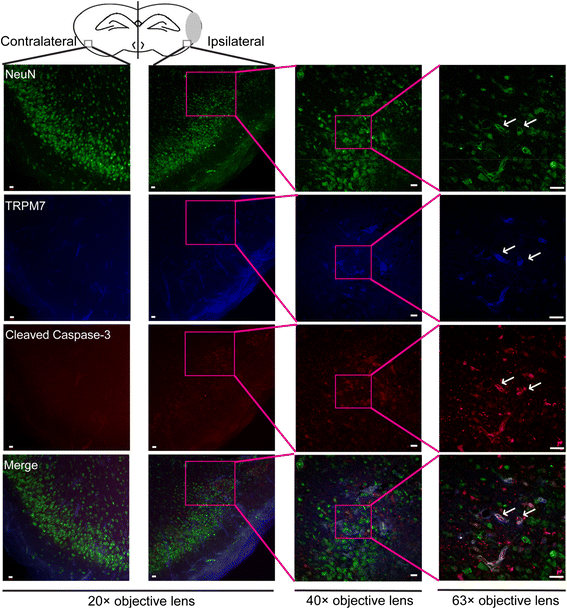
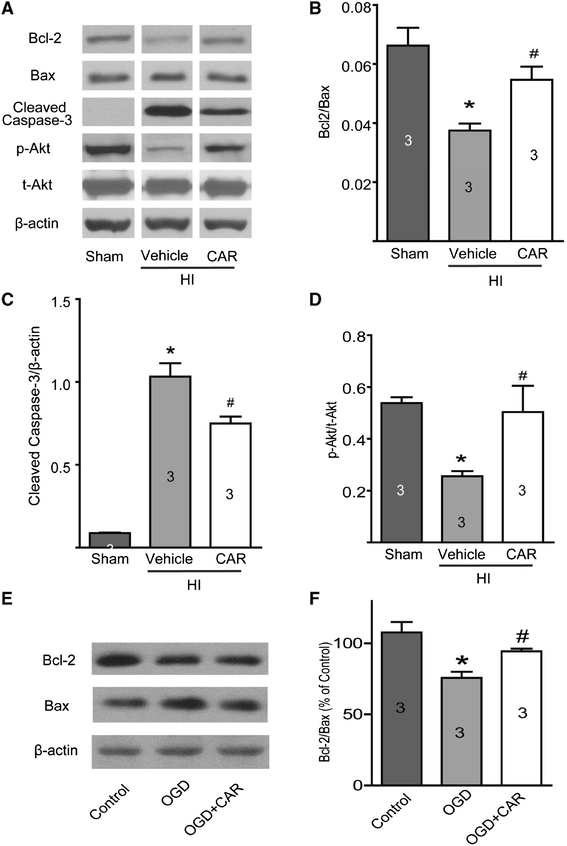
Results: Carvacrol inhibited TRPM7 current in HEK293 cells over-expressing TRPM7 and TRPM7-like current in hippocampal neurons in a dose-dependent manner. Carvacrol (>200 μM) reduced OGD-induced neuronal injury in cortical neurons. 24 hours after HI, TRPM7 protein level in the ipsilateral hemisphere was significantly higher than in the contralateral hemisphere. Carvacrol (30 and 50 mg/kg) pre-treatment reduced brain infarct volume 24 hours after HI in a dose-dependent manner. Carvacrol pre-treatment also improved neurobehavioral outcomes. Furthermore, animals pre-treated with carvacrol had fewer TUNEL-positive cells in the brain compared to vehicle-treated animals 3 days after HI. Carvacrol pre-treatment also increased Bcl-2/Bax and p-Akt/t-Akt protein ratios and decreased cleaved caspase-3 protein expression 24 hours after HI.
Conclusions: Carvacrol pre-treatment protects against neonatal hypoxic-ischemic brain injury by reducing brain infarct volume, promoting pro-survival signaling and inhibiting pro-apoptotic signaling, as well as improving behavioral outcomes. The neuroprotective effect may be mediated by the inhibition of TRPM7 channel function. Carvacrol is a potential drug development target for the treatment of neonatal stroke.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

