Single stream inertial focusing in a straight microchannel
- PMID: 25761900
- PMCID: PMC4388233
- DOI: 10.1039/c4lc01462f
Single stream inertial focusing in a straight microchannel
Abstract
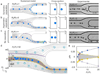
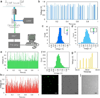
In the past two decades, microfluidics has become of great value in precisely aligning cells or microparticles within fluids. Microfluidic techniques use either external forces or sheath flow to focus particulate samples, and face the challenges of complex instrumentation design and limited throughput. The burgeoning field of inertial microfluidics brings single-position focusing functionality at throughput orders of magnitude higher than previously available. However, most inertial microfluidic focusers rely on cross-sectional flow-induced drag force to achieve single-position focusing, which inevitably complicates the device design and operation. In this work, we present an inertial microfluidic focuser that uses inertial lift force as the only driving force to focus microparticles into a single position. We demonstrate single-position focusing of different sized microbeads and cells with 95-100% efficiency, without the need for secondary flow, sheath flow or external forces. We further integrate this device with a laser counting system to form a sheathless flow cytometer, and demonstrated counting of microbeads with 2200 beads s(-1) throughput and 7% coefficient of variation. Cells can be completely recovered and remain viable after passing our integrated cytometry system. Our approach offers a number of benefits, including simplicity in fundamental principle and geometry, convenience in design, modification and integration, flexibility in focusing of different samples, high compatibility with real-world cellular samples as well as high-precision and high-throughput single-position focusing.
Figures




References
-
- Xuan X, Zhu J, Church C. Microfluid. Nanofluid. 2010;9:1–16.
-
- Nolan JP, Sklar LA. Nat. Biotechnol. 1998;16:633–638. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

