Deregulation of innate immune and inflammatory signaling in myelodysplastic syndromes
- PMID: 25761935
- PMCID: PMC4857136
- DOI: 10.1038/leu.2015.69
Deregulation of innate immune and inflammatory signaling in myelodysplastic syndromes
Abstract
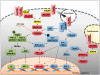
Myelodysplastic syndromes (MDSs) are a group of heterogeneous clonal hematologic malignancies that are characterized by defective bone marrow (BM) hematopoiesis and by the occurrence of intramedullary apoptosis. During the past decade, the identification of key genetic and epigenetic alterations in patients has improved our understanding of the pathophysiology of this disease. However, the specific molecular mechanisms leading to the pathogenesis of MDS have largely remained obscure. Recently, essential evidence supporting the direct role of innate immune abnormalities in MDS has been obtained, including the identification of multiple key regulators that are overexpressed or constitutively activated in BM hematopoietic stem and progenitor cells. Mounting experimental results indicate that the dysregulation of these molecules leads to abnormal hematopoiesis, unbalanced cell death and proliferation in patients' BM, and has an important role in the pathogenesis of MDS. Furthermore, there is compelling evidence that the deregulation of innate immune and inflammatory signaling also affects other cells from the immune system and the BM microenvironment, which establish aberrant associations with hematopoietic precursors and contribute to the MDS phenotype. Therefore, the deregulation of innate immune and inflammatory signaling should be considered as one of the driving forces in the pathogenesis of MDS. In this article, we review and update the advances in this field, summarizing the results from the most recent studies and discussing their clinical implications.
Figures


References
-
- Corey SJ, Minden MD, Barber DL, Kantarjian H, Wang JC, Schimmer AD. Myelodysplastic syndromes: the complexity of stem-cell diseases. Nat Rev Cancer. 2007;7:118–129. - PubMed
-
- Haferlach T. Molecular genetics in myelodysplastic syndromes. Leuk Res. 2012;36:1459–1462. - PubMed
-
- de Hollanda A, Beucher A, Henrion D, Ghali A, Lavigne C, Levesque H, et al. Systemic and immune manifestations in myelodysplasia: a multicenter retrospective study. Arthritis Care Res. 2011;63:1188–1194. - PubMed
-
- Hebbar M, Kozlowski D, Wattel E, Mastrini S, Dievart M, Duclos B, et al. Association between myelodysplastic syndromes and inflammatory bowel diseases. Report of seven new cases and review of the literature. Leukemia. 1997;11:2188–2191. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

