The Fe-S cluster-containing NEET proteins mitoNEET and NAF-1 as chemotherapeutic targets in breast cancer
- PMID: 25762074
- PMCID: PMC4378444
- DOI: 10.1073/pnas.1502960112
The Fe-S cluster-containing NEET proteins mitoNEET and NAF-1 as chemotherapeutic targets in breast cancer
Abstract
Identification of novel drug targets and chemotherapeutic agents is a high priority in the fight against cancer. Here, we report that MAD-28, a designed cluvenone (CLV) derivative, binds to and destabilizes two members of a unique class of mitochondrial and endoplasmic reticulum (ER) 2Fe-2S proteins, mitoNEET (mNT) and nutrient-deprivation autophagy factor-1 (NAF-1), recently implicated in cancer cell proliferation. Docking analysis of MAD-28 to mNT/NAF-1 revealed that in contrast to CLV, which formed a hydrogen bond network that stabilized the 2Fe-2S clusters of these proteins, MAD-28 broke the coordinative bond between the His ligand and the cluster's Fe of mNT/NAF-1. Analysis of MAD-28 performed with control (Michigan Cancer Foundation; MCF-10A) and malignant (M.D. Anderson-metastatic breast; MDA-MB-231 or MCF-7) human epithelial breast cells revealed that MAD-28 had a high specificity in the selective killing of cancer cells, without any apparent effects on normal breast cells. MAD-28 was found to target the mitochondria of cancer cells and displayed a surprising similarity in its effects to the effects of mNT/NAF-1 shRNA suppression in cancer cells, causing a decrease in respiration and mitochondrial membrane potential, as well as an increase in mitochondrial iron content and glycolysis. As expected, if the NEET proteins are targets of MAD-28, cancer cells with suppressed levels of NAF-1 or mNT were less susceptible to the drug. Taken together, our results suggest that NEET proteins are a novel class of drug targets in the chemotherapeutic treatment of breast cancer, and that MAD-28 can now be used as a template for rational drug design for NEET Fe-S cluster-destabilizing anticancer drugs.
Keywords: NEET proteins; iron-sulfur proteins; mitocan; mitochondria; rational drug design.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

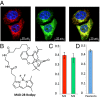
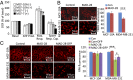


References
-
- Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281(5381):1309–1312. - PubMed
-
- Biasutto L, Dong LF, Zoratti M, Neuzil J. Mitochondrially targeted anti-cancer agents. Mitochondrion. 2010;10(6):670–681. - PubMed
-
- Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nat Rev Drug Discov. 2010;9(6):447–464. - PubMed
-
- Neuzil J, et al. Vitamin E analogues as a novel group of mitocans: Anti-cancer agents that act by targeting mitochondria. Mol Aspects Med. 2007;28(5-6):607–645. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

