Systemic and mucosal immunity in mice elicited by a single immunization with human adenovirus type 5 or 41 vector-based vaccines carrying the spike protein of Middle East respiratory syndrome coronavirus
- PMID: 25762305
- PMCID: PMC4515128
- DOI: 10.1111/imm.12462
Systemic and mucosal immunity in mice elicited by a single immunization with human adenovirus type 5 or 41 vector-based vaccines carrying the spike protein of Middle East respiratory syndrome coronavirus
Abstract
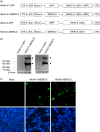
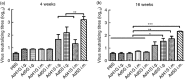
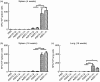
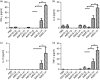
An ideal vaccine against mucosal pathogens such as Middle East respiratory syndrome coronavirus (MERS-CoV) should confer sustained, protective immunity at both systemic and mucosal levels. Here, we evaluated the in vivo systemic and mucosal antigen-specific immune responses induced by a single intramuscular or intragastric administration of recombinant adenoviral type 5 (Ad5) or type 41 (Ad41) -based vaccines expressing the MERS-CoV spike (S) protein. Intragastric administration of either Ad5-S or Ad41-S induced antigen-specific IgG and neutralizing antibody in serum; however, antigen-specific T-cell responses were not detected. In contrast, after a single intramuscular dose of Ad5-S or Ad41-S, functional antigen-specific T-cell responses were elicited in the spleen and pulmonary lymphocytes of the mice, which persisted for several months. Both rAd-based vaccines administered intramuscularly induced systemic humoral immune responses (neutralizing IgG antibodies). Our results show that a single dose of Ad5-S- or Ad41-S-based vaccines represents an appealing strategy for the control of MERS-CoV infection and transmission.
Keywords: Middle East respiratory syndrome coronavirus; adenoviral vector; immunity; spike protein; vaccine.
© 2015 John Wiley & Sons Ltd.
Figures

References
-
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–20. - PubMed
-
- Bermingham A, Chand MA, Brown CS, et al. Severe respiratory illness caused by a novel coronavirus, in a patient transferred to the United Kingdom from the Middle East. Euro Surveill. 2012;17:20290. - PubMed
-
- WHO. Middle East respiratory syndrome coronavirus (MERS-CoV), update. 26 December 2014.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

