Physiological and pharmacological modulation of the embryonic skeletal muscle calcium channel splice variant CaV1.1e
- PMID: 25762319
- PMCID: PMC4375451
- DOI: 10.1016/j.bpj.2015.01.026
Physiological and pharmacological modulation of the embryonic skeletal muscle calcium channel splice variant CaV1.1e
Abstract
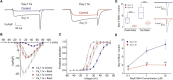
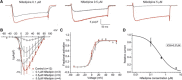
CaV1.1e is the voltage-gated calcium channel splice variant of embryonic skeletal muscle. It differs from the adult CaV1.1a splice variant by the exclusion of exon 29 coding for 19 amino acids in the extracellular loop connecting transmembrane domains IVS3 and IVS4. Like the adult splice variant CaV1.1a, the embryonic CaV1.1e variant functions as voltage sensor in excitation-contraction coupling, but unlike CaV1.1a it also conducts sizable calcium currents. Consequently, physiological or pharmacological modulation of calcium currents may have a greater impact in CaV1.1e expressing muscle cells. Here, we analyzed the effects of L-type current modulators on whole-cell current properties in dysgenic (CaV1.1-null) myotubes reconstituted with either CaV1.1a or CaV1.1e. Furthermore, we examined the physiological current modulation by interactions with the ryanodine receptor using a chimeric CaV1.1e construct in which the cytoplasmic II-III loop, essential for skeletal muscle excitation-contraction coupling, has been replaced with the corresponding but nonfunctional loop from the Musca channel. Whereas the equivalent substitution in CaV1.1a had abolished the calcium currents, substitution of the II-III loop in CaV1.1e did not significantly reduce current amplitudes. This indicates that CaV1.1e is not subject to retrograde coupling with the ryanodine receptor and that the retrograde coupling mechanism in CaV1.1a operates by counteracting the limiting effects of exon 29 inclusion on the current amplitude. Pharmacologically, CaV1.1e behaves like other L-type calcium channels. Its currents are substantially increased by the calcium channel agonist Bay K 8644 and inhibited by the calcium channel blocker nifedipine in a dose-dependent manner. With an IC50 of 0.37 μM for current inhibition by nifedipine, CaV1.1e is a potential drug target for the treatment of myotonic dystrophy. It might block the excessive calcium influx resulting from the aberrant expression of the embryonic splice variant CaV1.1e in the skeletal muscles of myotonic dystrophy patients.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Melzer W., Herrmann-Frank A., Lüttgau H.C. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochim. Biophys. Acta. 1995;1241:59–116. - PubMed
-
- Nakai J., Dirksen R.T., Allen P.D. Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature. 1996;380:72–75. - PubMed
-
- Perez-Reyes E., Kim H.S., Birnbaumer L. Induction of calcium currents by the expression of the alpha 1-subunit of the dihydropyridine receptor from skeletal muscle. Nature. 1989;340:233–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

