Removal of perineuronal nets in the medial prefrontal cortex impairs the acquisition and reconsolidation of a cocaine-induced conditioned place preference memory
- PMID: 25762666
- PMCID: PMC4355195
- DOI: 10.1523/JNEUROSCI.3592-14.2015
Removal of perineuronal nets in the medial prefrontal cortex impairs the acquisition and reconsolidation of a cocaine-induced conditioned place preference memory
Erratum in
- J Neurosci. 2015 May 27;35(21):8376
Abstract
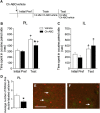
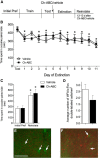
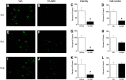
Pyramidal neurons in the medial prefrontal cortex (mPFC) critically contribute to cocaine-seeking behavior in humans and rodents. Activity of these neurons is significantly modulated by GABAergic, parvalbumin-containing, fast-spiking interneurons, the majority of which are enveloped by specialized structures of extracellular matrix called perineuronal nets (PNNs), which are integral to the maintenance of many types of plasticity. Using a conditioned place preference (CPP) procedure, we found that removal of PNNs primarily from the prelimbic region of the mPFC of adult, male, Sprague Dawley rats impaired the acquisition and reconsolidation of a cocaine-induced CPP memory. This impairment was accompanied by a decrease in the number of c-Fos-positive cells surrounded by PNNs. Following removal of PNNs, the frequency of inhibitory currents in mPFC pyramidal neurons was decreased; but following cocaine-induced CPP, both frequency and amplitude of inhibitory currents were decreased. Our findings suggest that cocaine-induced plasticity is impaired by removal of prelimbic mPFC PNNs and that PNNs may be a therapeutic target for disruption of cocaine CPP memories.
Keywords: cocaine; conditioned place preference; memory; perineuronal net.
Copyright © 2015 the authors 0270-6474/15/354190-13$15.00/0.
Figures




References
-
- Brückner G, Brauer K, Härtig W, Wolff JR, Rickmann MJ, Derouiche A, Delpech B, Girard N, Oertel WH, Reichenbach A. Perineuronal nets provide a polyanionic, glia-associated form of microenvironment around certain neurons in many parts of the rat brain. Glia. 1993;8:183–200. doi: 10.1002/glia.440080306. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
