Excitation of tuberoinfundibular dopamine neurons by oxytocin: crosstalk in the control of lactation
- PMID: 25762669
- PMCID: PMC6605300
- DOI: 10.1523/JNEUROSCI.2633-14.2015
Excitation of tuberoinfundibular dopamine neurons by oxytocin: crosstalk in the control of lactation
Abstract
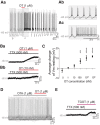
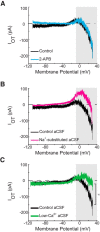
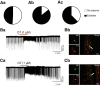
Milk production in the nursing mother is induced by the hormone prolactin. Its release from the anterior pituitary is generally under tonic inhibition by neuroendocrine tuberoinfundibular dopamine (TIDA) neurons of the arcuate nucleus. Successful nursing, however, requires not only production but also ejection of breast milk. This function is supported by the hormone oxytocin. Here we explored the possibility that interaction between these functionally complementary hormones is mediated by TIDA neurons. First, whole-cell patch-clamp recordings were performed on prepubertal male rat hypothalamic slices, where TIDA neurons can be identified by a robust and rhythmic membrane potential oscillation. Oxytocin induced a switch of this rhythmic activity to tonic discharge through a depolarization involving direct actions on TIDA neurons. The depolarization is sensitive to blockade of the oxytocin receptor and is mediated by a voltage-dependent inward current. This inward current has two components: a canonical transient receptor potential-like conductance in the low-voltage range, and in the high-voltage range, a Ca(2+)-dependent component. Finally, whole-cell and loose-patch recordings were also performed on slices from virgin and lactating female rats to evaluate the relevance of these findings for nursing. In these preparations, oxytocin was found to excite TIDA neurons, identified by their expression of tyrosine hydroxylase. These findings suggest that oxytocin can modulate prolactin secretion by exciting TIDA neurons, and that this may serve as a feedforward inhibition of prolactin release.
Keywords: arcuate nucleus; oscillation; prolactin.
Copyright © 2015 the authors 0270-6474/15/354229-09$15.00/0.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
