Representation of accumulating evidence for a decision in two parietal areas
- PMID: 25762677
- PMCID: PMC4355201
- DOI: 10.1523/JNEUROSCI.2451-14.2015
Representation of accumulating evidence for a decision in two parietal areas
Abstract
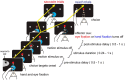
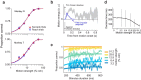
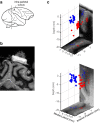
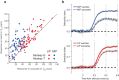
Decisions are often made by accumulating evidence for and against the alternatives. The momentary evidence represented by sensory neurons is accumulated by downstream structures to form a decision variable, linking the evolving decision to the formation of a motor plan. When decisions are communicated by eye movements, neurons in the lateral intraparietal area (LIP) represent the accumulation of evidence bearing on the potential targets for saccades. We now show that reach-related neurons from the medial intraparietal area (MIP) exhibit a gradual modulation of their firing rates consistent with the representation of an evolving decision variable. When decisions were communicated by saccades instead of reaches, decision-related activity was attenuated in MIP, whereas LIP neurons were active while monkeys communicated decisions by saccades or reaches. Thus, for decisions communicated by a hand movement, a parallel flow of sensory information is directed to parietal areas MIP and LIP during decision formation.
Keywords: LIP; MIP; decision-making; reaches; saccades; sensory.
Copyright © 2015 the authors 0270-6474/15/354306-13$15.00/0.
Figures





Comment in
-
Cognitive and action-based aspects of developing decisions in parietal cortex.J Neurosci. 2015 Jun 3;35(22):8382-3. doi: 10.1523/JNEUROSCI.1181-15.2015. J Neurosci. 2015. PMID: 26041907 Free PMC article. No abstract available.
-
Is There a General Role for the Monkey Oculomotor System in Perceptual Decision-Making?J Neurosci. 2015 Jul 8;35(27):9783-5. doi: 10.1523/JNEUROSCI.1818-15.2015. J Neurosci. 2015. PMID: 26156981 Free PMC article. No abstract available.
References
-
- Barlow H. The cognitive neurosciences. Cambridge, MA: Massachusetts Institute of Technology; 1995. The neuron doctrine in perception; pp. 415–435.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
