Hematopoietic mobilization: Potential biomarker of response to natalizumab in multiple sclerosis
- PMID: 25762712
- PMCID: PMC4395887
- DOI: 10.1212/WNL.0000000000001454
Hematopoietic mobilization: Potential biomarker of response to natalizumab in multiple sclerosis
Abstract
Objective: To ascertain the mobilization from the bone marrow and the functional relevance of the increased number of circulating hematopoietic stem and progenitor cells (HSPC) induced by the anti-α-4 integrin antibody natalizumab in patients with multiple sclerosis (MS).
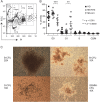
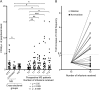
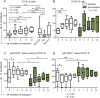
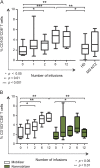
Methods: We evaluated CD45(low)CD34+ HSPC frequency by flow cytometry in blood from 45 natalizumab-treated patients (12 of whom were prospectively followed during the first year of treatment as part of a pilot cohort and 16 prospectively followed for validation), 10 untreated patients with MS, and 24 healthy donors. In the natalizumab-treated group, we also assessed sorted HSPC cell cycle status, T- and B-lymphocyte subpopulation frequencies (n = 29), and HSPC differentiation potential (n = 10).
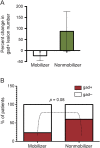
Results: Natalizumab-induced circulating HSPC were predominantly quiescent, suggesting recent mobilization from the bone marrow, and were capable of differentiating ex vivo. Circulating HSPC numbers were significantly increased during natalizumab, but heterogeneously, allowing the stratification of mobilizer and nonmobilizer subgroups. Nonmobilizer status was associated with persistence of disease activity during treatment. The frequency of B cells and CD103+CD8+ regulatory T cells persistently increased, more significantly in mobilizer patients, who also showed a specific naive/memory B-cell profile.
Conclusions: The data suggest that natalizumab-induced circulating HSPC increase is the result of true mobilization from the bone marrow and has clinical and immunologic relevance. HSPC mobilization, associated with clinical remission and increased proportion of circulating B and regulatory T cells, may contribute to the treatment's mode of action; thus, HSPC blood counts could represent an early biomarker of responsiveness to natalizumab.
© 2015 American Academy of Neurology.
Figures
References
-
- Rose DM, Alon R, Ginsberg MH. Integrin modulation and signaling in leukocyte adhesion and migration. Immunol Rev 2007;218:126–134. - PubMed
-
- Miller DH, Soon D, Fernando KT, et al. MRI outcomes in a placebo-controlled trial of natalizumab in relapsing MS. Neurology 2007;68:1390–1401. - PubMed
-
- Polman CH, O'Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006;354:899–910. - PubMed
-
- Radue EW, Stuart WH, Calabresi PA, et al. Natalizumab plus interferon beta-1a reduces lesion formation in relapsing multiple sclerosis. J Neurol Sci 2010;292:28–35. - PubMed
-
- Phillips JT, Giovannoni G, Lublin FD, et al. Sustained improvement in expanded disability status scale as a new efficacy measure of neurological change in multiple sclerosis: treatment effects with natalizumab in patients with relapsing multiple sclerosis. Mult Scler 2011;17:970–979. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
