The lung mycobiome: an emerging field of the human respiratory microbiome
- PMID: 25762987
- PMCID: PMC4327734
- DOI: 10.3389/fmicb.2015.00089
The lung mycobiome: an emerging field of the human respiratory microbiome
Abstract
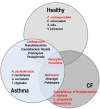
The lung microbiome, which is believed to be stable or at least transient in healthy people, is now considered as a poly-microorganism component contributing to disease pathogenesis. Most research studies on the respiratory microbiome have focused on bacteria and their impact on lung health, but there is evidence that other non-bacterial organisms, comprising the viruses (virome) and fungi (mycobiome), are also likely to play an important role in healthy people as well as in patients. In the last few years, the lung mycobiome (previously named the fungal microbiota or microbiome) has drawn closer attention. There is growing evidence that the lung mycobiome has a significant impact on clinical outcome of chronic respiratory diseases (CRD) such as asthma, chronic obstructive pulmonary disease, cystic fibrosis, and bronchiectasis. Thanks to advances in culture independent methods, especially next generation sequencing, a number of fungi not detected by culture methods have been molecularly identified in human lungs. It has been shown that the structure and diversity of the lung mycobiome vary in different populations (healthy and different diseased individuals) which could play a role in CRD. Moreover, the link between lung mycobiome and different biomes of other body sites, especially the gut, has also been unraveled. By interacting with the bacteriome and/or virome, the respiratory mycobiome appears to be a cofactor in inflammation and in the host immune response, and therefore may contribute to the decline of the lung function and the disease progression. In this review, we report the recent limited explorations of the human respiratory mycobiome, and discuss the mycobiome's connections with other local microbial communities, as well as the relationships with the different biomes of other body sites. These studies suggest several outlooks for this understudied emerging field, which will certainly call for a renewal of our understanding of pulmonary diseases.
Keywords: chronic respiratory disease; fungal microbiota; lung; microbiome; mycobiota; respiratory mycobiome.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

